SCIENTIFIC ACHIEVEMENT
With key data from the Advanced Light Source (ALS), researchers explained how a new, low-cost electrocatalyst enables an important oxygen reaction to proceed at an ultrafast rate.
SIGNIFICANCE AND IMPACT
The work provides rational guidance for the development of better electrocatalysts for applications such as hydrogen-fuel production and long-range batteries for electric vehicles.
Enhancing the oxygen evolution reaction
The oxygen evolution reaction (OER) is the electrochemical mechanism at the heart of many processes relevant to energy storage and conversion, including the splitting of water to generate hydrogen fuel and the operation of proposed long-range batteries for electric vehicles. Because the OER rate is a limiting factor in such processes, highly active OER electrocatalysts with long-term stability are being sought to increase reaction rates, reduce energy losses, and improve cycling stability. Catalysts incorporating rare and expensive materials such as iridium and ruthenium exhibit good performance, but an easily prepared, efficient, and durable OER catalyst based on earth-abundant elements is still needed for large-scale applications.
Key insight: shorter O-O bonds
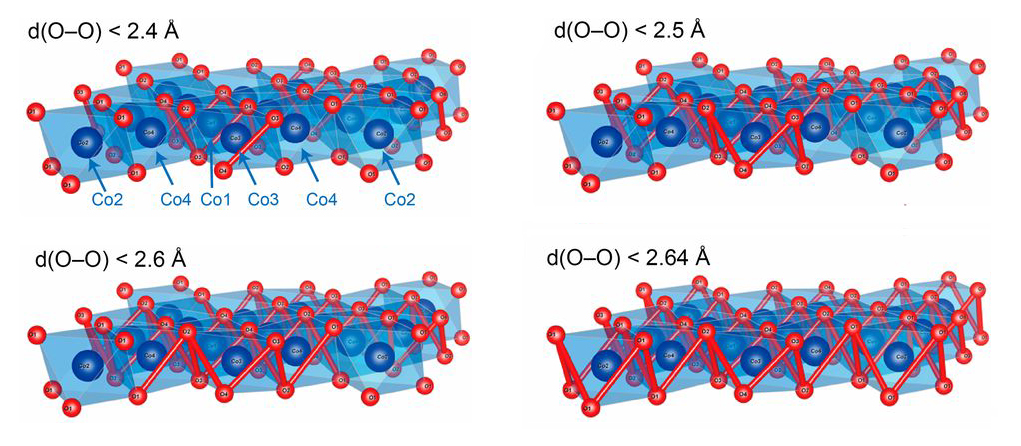
In an earlier study, a group led by John Goodenough (2019 Nobel laureate in chemistry) measured the OER activities of two compounds with similar structures: CaCoO3 and SrCoO3. They found that the CaCoO3 exhibited higher OER activity, which they attributed to its shorter oxygen–oxygen (O-O) bonds. Inspired by this, members of the Goodenough group have now analyzed a metallic layered oxide, Na0.67CoO2, which has an even more compact structure than CaCoO3.
X-ray diffraction (XRD) experiments performed at the Advanced Photon Source (APS) confirmed that the shortest O-O separation in Na0.67CoO2 is 2.30 Å, compared to 2.64 Å for CaCoO3. The researchers then compared the OER performance of Na0.67CoO2 with IrO2, Co3O4, and Co(OH)2. They found that Na0.67CoO2 exhibited the highest current density, the lowest overpotential (a measure of thermodynamic energy loss), and the most favorable Tafel slope (sensitivity of the electric current to applied potential). The Na0.67CoO2 also showed excellent stability under typical operating conditions.
A probe of valence and spin
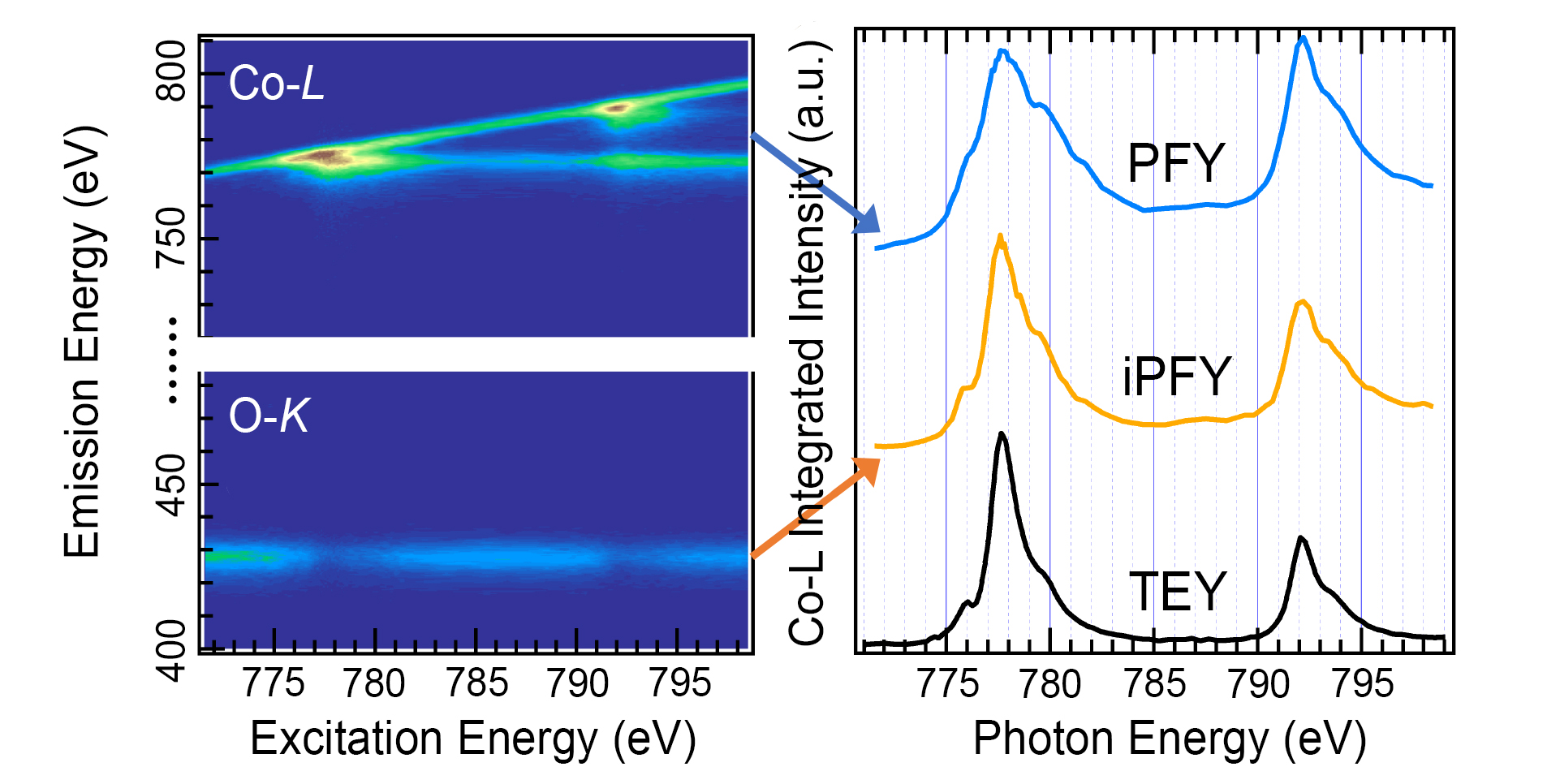
To better understand how O-O bond lengths affect the OER, the researchers probed the Co valence and spin states using soft x-ray absorption spectroscopy (XAS) and mapping of resonant inelastic x-ray scattering (mRIXS) at ALS Beamline 8.0.1. With spectra extracted from the mRIXS data in various ways, both the chemical and spin states of the Co, in the bulk and on the surface, were derived and quantitatively analyzed. Thus, the mean valence state of the Co was found to be 3.3+, and the ratio of the of the L3 and L2 edges revealed that the Co electron configuration was in a low-spin state, which is consistent with a more compact structure and intrinsically beneficial to OER stability.
Competing reaction rates
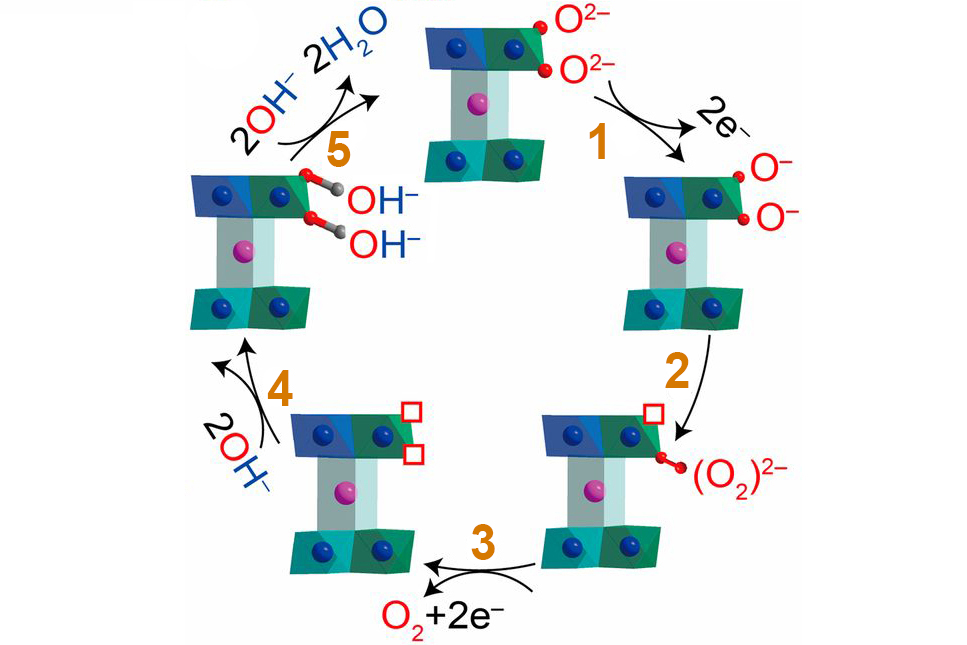
Based on the insights revealed by this new data, the researchers proposed an alternative OER mechanism to explain the ultrafast performance of Na0.67CoO2. Whereas conventional OER is initiated by interactions between surface anions and OH– from the electrolyte solution, the new pathway starts with interactions between oxygen ions in neighboring CoO2 layers. The reaction rate of this alternate pathway increases steeply as the O-O separation decreases, quickly outpacing the conventional route when O-O bonds are short. In total, the results demonstrate an invaluable array of tools and techniques that will greatly enhance future efforts to rationally design low-cost alternatives to precious-metal OER electrocatalysts.
Contacts: Jinpeng Wu and Wanli Yang
Researchers: H. Wang, A. Dolocan, Y. Li, N. Wu, K. Park, S. Xin, and J.B. Goodenough (University of Texas at Austin); J. Wu and W. Yang (ALS); X. Lü (Center for High Pressure Science and Technology Advanced Research, Shanghai); and M. Lei (Beijing University of Posts and Telecommunications).
Funding: U.S. Department of Energy (DOE), Office of Energy Efficiency and Renewable Energy, and the Robert A. Welch Foundation. Operation of the ALS is supported by the DOE Office of Science, Basic Energy Sciences Program.
Publication: H. Wang, J. Wu, A. Dolocan, Y. Li, X. Lü, N. Wu, K. Park, S. Xin, M. Lei, W. Yang, and J.B. Goodenough, “Short O–O separation in layered oxide Na0.67CoO2 enables an ultrafast oxygen evolution reaction,” PNAS 116, 23473 (2019), doi: 10.1073/pnas.1901046116.
ALS SCIENCE HIGHLIGHT #415