SCIENTIFIC ACHIEVEMENT
Researchers used the Advanced Light Source (ALS) to unravel how water helps catalyze the conversion of methane, the main component of natural gas, into methanol, a liquid fuel.
SIGNIFICANCE AND IMPACT
The work supports the efficient production of methanol and other useful chemicals and could help reduce the amount of greenhouse gases released by the flaring and venting of methane.
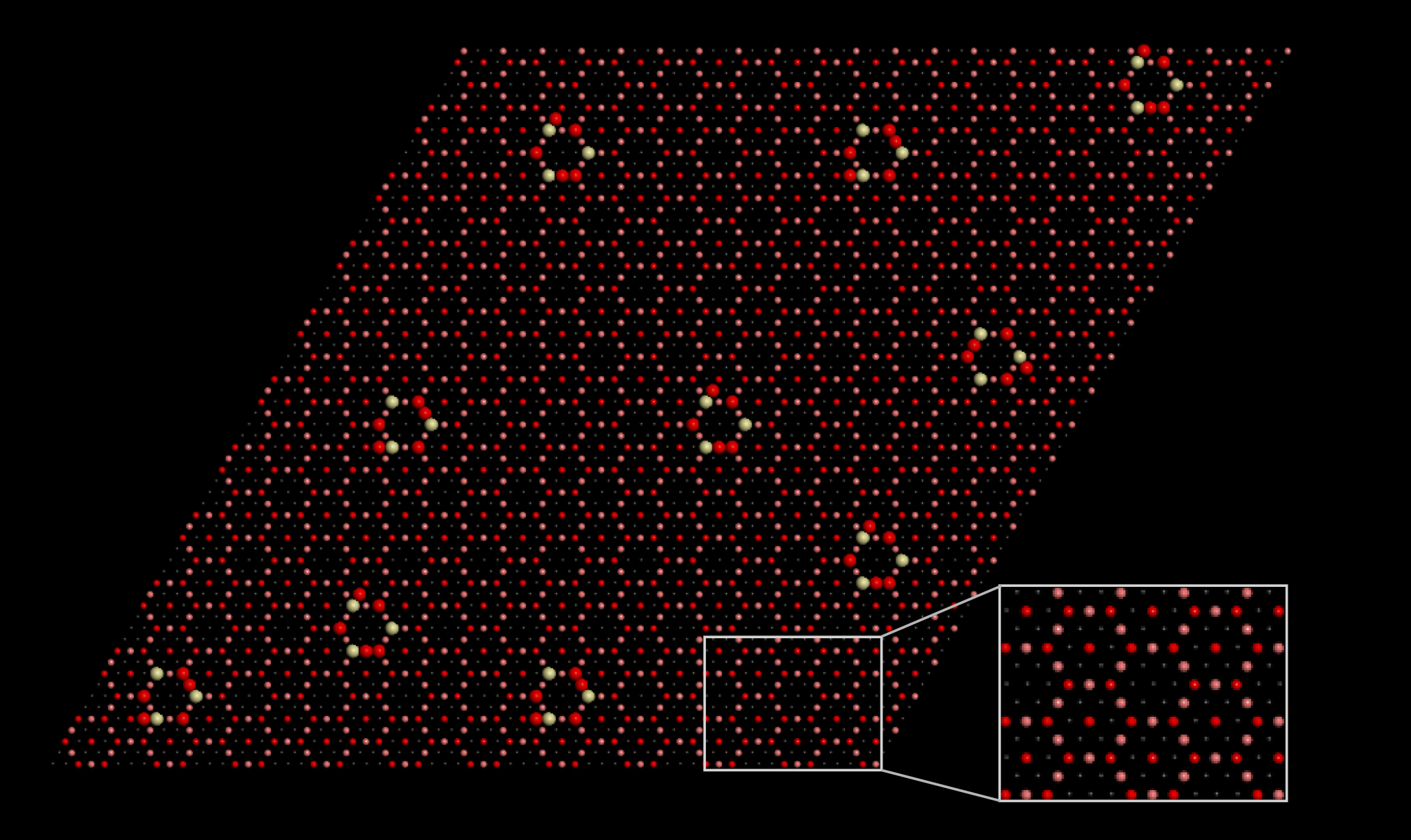
Making the most of methane
Natural gas has fast become a cheap and abundant resource, transforming the energy landscape of the United States. Its main component, methane gas (CH4), can be converted into a liquid fuel, methanol (CH3OH), which in turn can be used as a feedstock for other commodity chemicals. However, the unfavorable economics of capturing, transporting, and processing the methane released during oil drilling means that significant amounts of it get burned off or vented, contributing harmful greenhouse gases to the atmosphere.
The direct conversion of methane to methanol would make the recovery of natural gas more profitable and sustainable. However, this conversion is very challenging, because methane’s strong C-H bonds require high temperatures to break. Yet, if the temperature is too high, the reaction will produce unwanted byproducts—CO or CO2. Researchers have thus been searching for catalysts that will work at low temperatures and with high selectivity for methanol.
Inverted ceria catalyst
Traditionally, industrial catalysts have consisted of a small amount of metal dispersed on a metal oxide support. In “inverted” catalysts, oxide nanoparticles are deposited on a metal substrate. Such systems offer more opportunities for tuning: small size, strong oxide–metal interactions, and abundant interfaces where reactions tend to occur.
In earlier work, a group of researchers from Brookhaven National Laboratory discovered a promising inverted catalyst, CeO2-Cu2O, that converts methane to methanol with high (70%) selectivity at room temperature in the presence of water. The group studied the material’s morphology using various microscopic probes and predicted its step-by-step molecular rearrangements using Monte Carlo simulations and density functional theory (DFT) calculations, performed in part at Berkeley Lab’s National Energy Research Scientific Computing Center (NERSC).
Ambient reality check
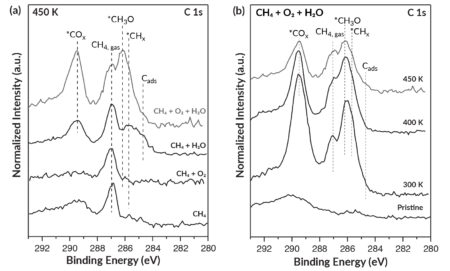
To confirm the predictions and obtain direct evidence for the essential role of water, the researchers used ambient-pressure x-ray photoelectron spectroscopy (APXPS) at ALS Beamline 9.3.2. The beamline provides tunable soft x-rays that are ideally suited for APXPS measurements, with high flux, excellent energy resolution, and an optimal energy range for probing electronic signatures of interest.
The samples, which consisted of inverted CeO2-Cu2O catalysts on Cu(111), were exposed to various combinations of methane gas, oxygen gas, and water vapor. Consistent with predictions, the results revealed that methoxy groups (CH3O)—immediate precursors of methanol—formed on the catalytic surface in the presence of water. The appearance of the surface methoxy groups was strongly correlated with high selectivity for the production of methanol.
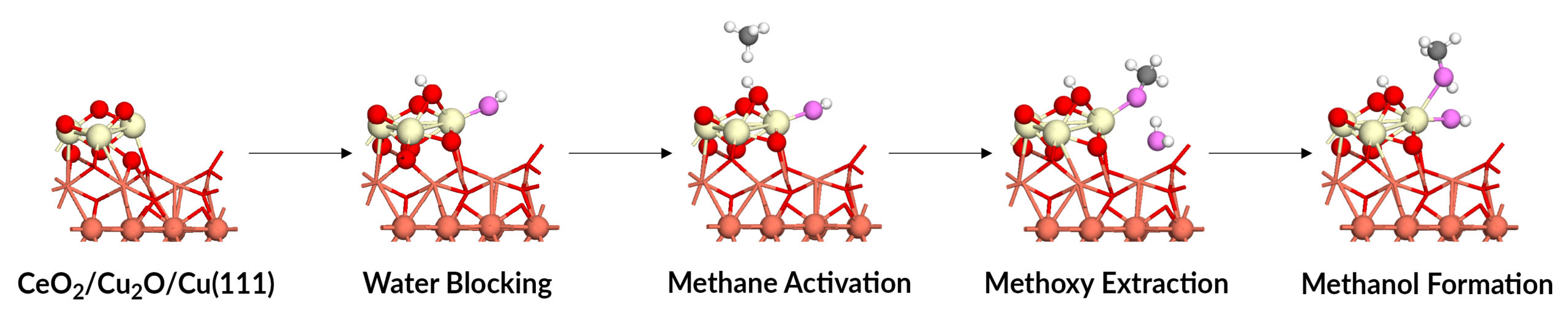
Triple action of water
The APXPS data, together with the theory and simulation work, established that water plays a key triple role in methane-to-methanol conversion: blocking an unwanted reaction, activating the desired reaction, and extracting the final product. Water molecules preferentially dissociate over the active cerium ions, blocking O-O bond cleavage that would otherwise dehydrogenate methoxy groups to CO and CO2. The dissociated water molecules formed adsorbed hydroxyl groups that directly converted methane to methanol. And water adsorption displaced the produced methanol into the gas phase, enabling it to be extracted. Overall, the study establishes design principles for optimizing the catalyst’s active site in the future and extending the results to highly active chemical processes in general.
Contact: Sanjaya Senanayake
Researchers: Z. Liu, R.M. Palomino, N. Rui, M. Mahapatra, and S.D. Senanayake (Brookhaven National Laboratory); E. Huang, I. Orozco, and W. Liao (Stony Brook University); T. Duchoň (Peter-Grünberg-Institut, Germany); S. Nemšák (ALS); D.C. Grinter (Diamond Light Source, UK); and P. Liu and J.A. Rodriguez (Brookhaven National Laboratory and Stony Brook University).
Funding: National Science Foundation and U.S. Department of Energy, Office of Science, Basic Energy Sciences program (DOE BES). Operation of the ALS is supported by DOE BES.
Publication: Z. Liu, E. Huang, I. Orozco, W. Liao, R.M. Palomino, N. Rui, T. Duchoň, S. Nemšák, D.C. Grinter, M. Mahapatra, P. Liu, J.A. Rodriguez, and S.D. Senanayake, “Water-promoted interfacial pathways in methane oxidation to methanol on a CeO2-Cu2O catalyst,” Science 368, 513 (2020), doi:10.1126/science.aba5005.
ALS SCIENCE HIGHLIGHT #424