SCIENTIFIC ACHIEVEMENT
Using a combination of tools at the Advanced Light Source (ALS) and other facilities, researchers probed specific mechanisms affecting the efficiency of catalysts for CO-to-CO2 conversion.
SIGNIFICANCE AND IMPACT
The work brings us closer to the rational design of more effective catalysts for cleaning up toxic CO exhaust and advances our understanding of fundamental catalytic reactions.
A better way to clear the air
The conversion of carbon monoxide (CO) into carbon dioxide (CO2) is important for cleaning up exhaust gases—mostly from vehicles that burn fossil fuels but also from stationary sources such as power plants and refineries. Platinum (Pt) catalysts are widely used to process CO into CO2, but the high cost of platinum-group metals has driven efforts to replace these materials with more earth-abundant alternatives.
One of the most promising ways of reducing the cost of metal catalysts is to mix them with oxygen-containing (oxide) compounds. In a previous investigation, researchers tested various mesoporous transition-metal oxides and loaded them with Pt nanoparticles. Each oxide exhibited enhanced CO-to-CO2 conversion activity, but the enhancement was largest for cobalt oxide.
Operando study of an inverse catalyst
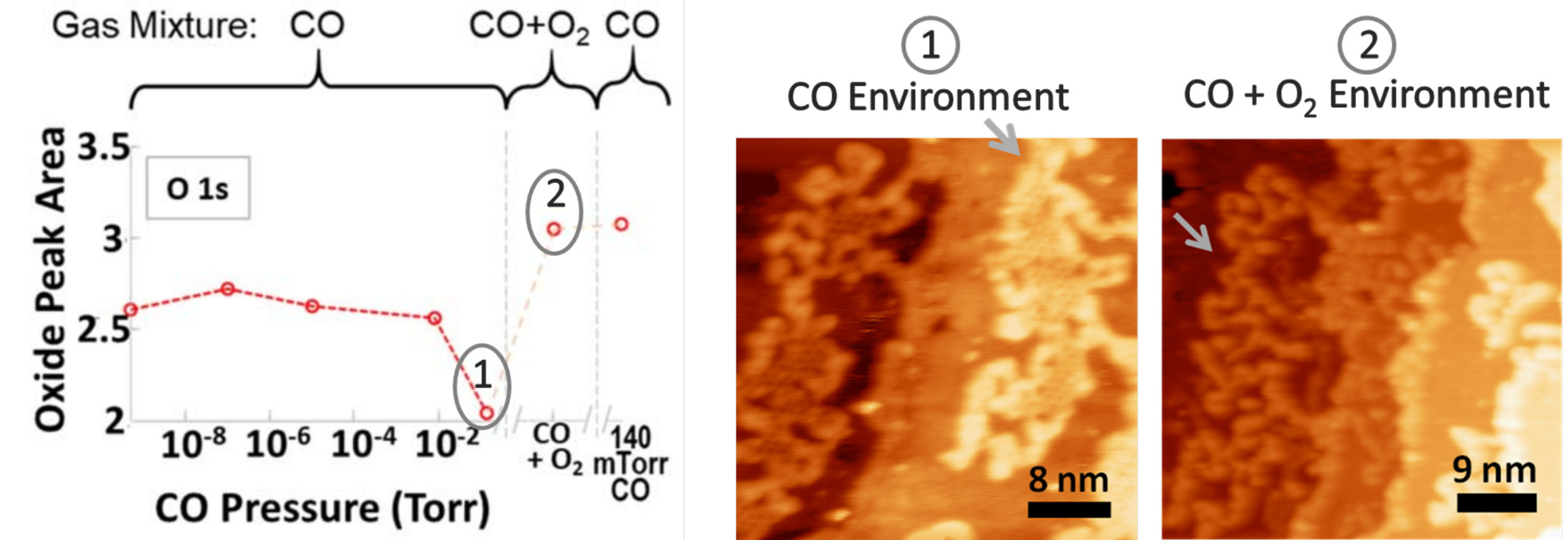
To understand the origin of this enhanced activity, the researchers investigated the atomic and electronic structure of a Pt/cobalt oxide (CoO) model catalyst under operating conditions (i.e., in the presence of CO gas and/or oxygen). While their earlier study looked at platinum nanoparticles on oxide surfaces, in this study, the researchers used so-called “inverse” catalysts, where the oxide was formed on a platinum surface. The well-defined platinum surfaces enabled them to correlate the nanoscale surface structure, revealed by high-pressure scanning tunneling microscopy (HPSTM), with surface oxidation states, measured using ambient-pressure x-ray photoelectron spectroscopy (APXPS) at NSLS‑II and the ALS.
APXPS at ALS Beamline 11.0.2 enables the identification of surface molecules and their chemical states as reactions take place in environments of oxygen and CO gas at pressures as high as several Torr. Thus, the researchers could intentionally modify the sample environment to carefully distinguish each spectroscopic feature and understand individual processes. Measurements at NSLS‑II also probed these phenomenona on CoO films of different thicknesses. The results were combined with theoretical calculations performed at UCLA and the University of Central Florida.
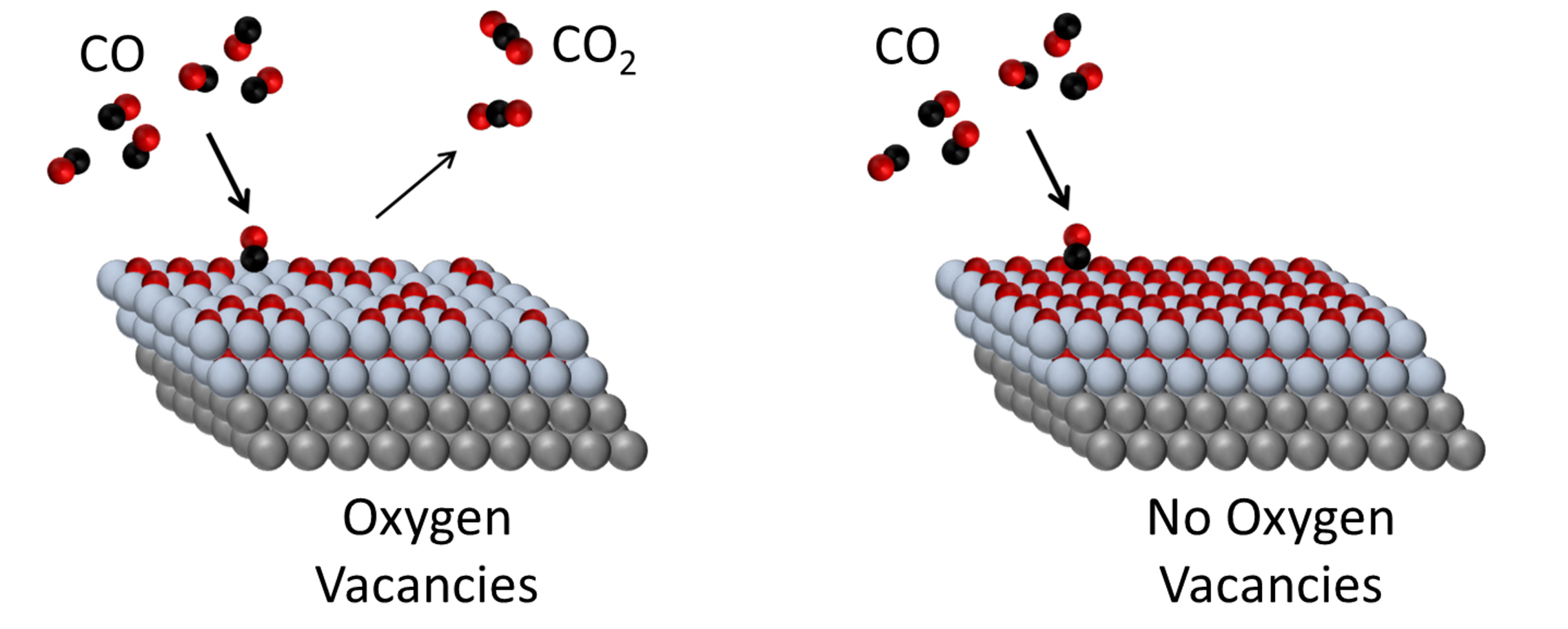
Oxygen vacancies are key to activation
The researchers found that CoO, a material that’s generally considered relatively inefficient for CO2 formation, could be made active for the reaction by removing some of its oxygen atoms, creating oxygen vacancies. With that modification, this oxide was able to drive CO2 formation at significantly lower temperatures, making it a much more energy-efficient reaction.
The investigations also revealed that CO can react with some oxide sites to form a highly stable carbonate (CO3) bound to the surface. The nanometer-scale structures observed with HPSTM indicated that the stable carbonate is formed preferentially at the edges of CoO islands, where both Pt and CoO were exposed. Edge sites are often considered to be active sites, but for this catalyst, that’s not the case.
The findings demonstrate a new pathway to efficient CO oxidation and suggest that complete, one-monolayer CoO films with no exposed Pt may benefit from less carbonate formation, to produce more active catalysts. The researchers are currently engaging in a series of further experiments to understand how the oxide interacts with the metal surface, and what effects that interaction has on the catalytic activity.
Contact: Heath Kersell
Researchers: H. Kersell, B. Eren, and C.H. Wu (Berkeley Lab); Z. Hooshmand, D. Le, and T.S. Rahman (Univ. of Central Florida); G. Yan, H. Nguyen, and P. Sautet (Univ. of California, Los Angeles); I. Waluyo and A. Hunt (Brookhaven National Laboratory); S. Nemšák (ALS); and G. Somorjai and M. Salmeron (Berkeley Lab and Univ. of California, Berkeley).
Funding: U.S. Department of Energy, Office of Science, Basic Energy Sciences program (DOE BES), National Science Foundation. Operation of the ALS is supported by DOE BES.
Publication: H. Kersell, Z. Hooshmand, G. Yan, D. Le, H. Nguyen, B. Eren, C.H. Wu, I. Waluyo, A. Hunt, S. Nemšák, G. Somorjai, T.S. Rahman, P. Sautet, and M. Salmeron, “CO Oxidation Mechanisms on CoOx-Pt Thin Films,” J. Am. Chem. Soc. 142, 8312 (2020), doi:10.1021/jacs.0c01139.
ALS SCIENCE HIGHLIGHT #429