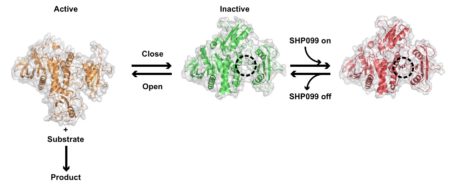
Mutations in the proteins that regulate cellular processes such as growth, division, and death are often linked to cancer and other diseases. The proper function of one of these proteins, SHP2, depends on maintaining equilibrium in a structural tug-of-war between an open (active) and a closed (inactive) arrangement. Normally, SHP2 undergoes structural transitions multiple times every second to maintain the equilibrium. But a single mutation can force SHP2 to stay active, leading to uncontrolled cell division and lethal consequences. In this study, a team of researchers from Brandeis University used a combination of approaches that focused on nuclear magnetic resonance and x-ray crystallography to elucidate the healthy and mutated forms of SHP2, the dynamic interchange between open and closed structures, and how SHP2 interacts with certain cancer drugs.
X-ray crystallography performed at Advanced Light Source (ALS) Beamlines 8.2.1 and 8.2.2 (part of the Berkeley Center for Structural Biology) as well as the Stanford Synchrotron Radiation Lightsource (SSRL) revealed that a leukemia-associated mutation shifts the protein angles, forcing it to remain in the open arrangement and activating cell division processes that lead to cancer. The researchers also clarified that a recently developed cancer drug, SHP099, attaches to the closed, inactive form of SHP2, locking it in this conformation. However, since mutations in SHP2 shift the equilibrium toward the open state, deactivating SHP2 requires more drug than would be medicinally sustainable. Now understanding more about SHP2’s structure, equilibrium, and how the SHP099 drug binds to SHP2, researchers can develop better approaches or more potent drugs to stem the growth of deadly cancers.
R. A. P. Pádua, Y. Sun, I. Marko, W. Pitsawong, J. B. Stiller, R. Otten, and D. Kern, “Mechanism of activating mutations and allosteric drug inhibition of the phosphatase SHP2,”Nat. Commun. 9, 4507 (2018), doi:10.1038/s41467-018-06814-w.