SCIENTIFIC ACHIEVEMENT
Scientists have detected a novel chemical state of the element manganese that was first proposed about 90 years ago.
SIGNIFICANCE AND IMPACT
The discovery enables the design of a high-performance, low-cost battery that, according to its developers, outperforms Department of Energy goals on cost and cycle life for grid-scale energy storage.
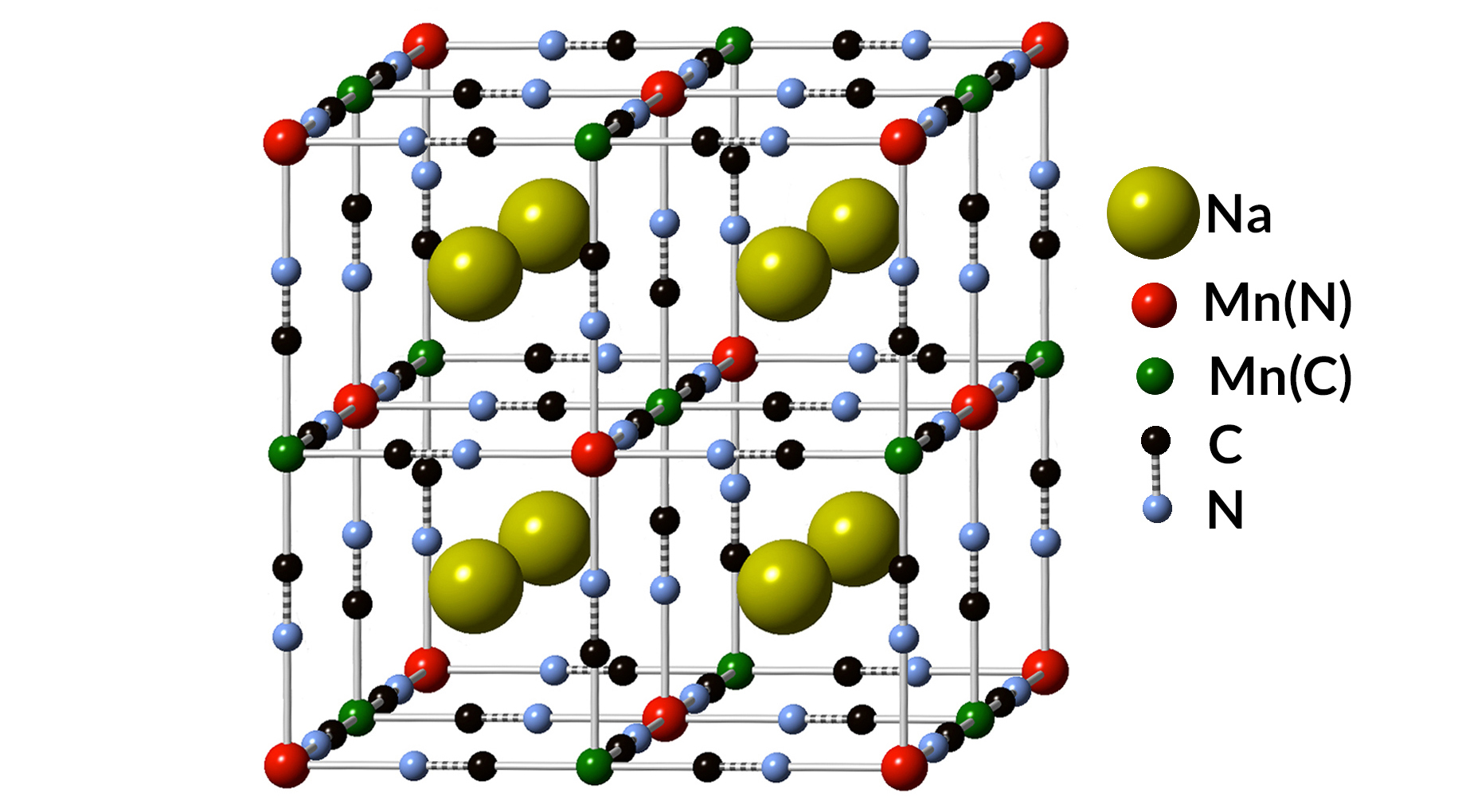
Stabilizing the energy grid
The widespread deployment of renewable energy sources such as solar and wind power destabilizes the electric grid because conventional power-generation systems cannot ramp quickly enough to balance the power variations from these intermittent sources. Storing energy in batteries could help to even things out, but the cost of most existing technologies—including lithium-ion batteries—is significant, hindering grid-scale applications.
Emerging storage technologies such as aqueous sodium (Na) systems offer low costs for long-duration storage, but they do not have the charge/discharge rates needed to balance volatile power generation. In particular, it remains a critical challenge to develop a stable negative electrode (anode) for high-rate Na-ion battery systems.
A battery breakthrough
Compared with the relatively mature designs of anodes used in Li-ion batteries, anodes for Na-ion batteries remain an active focus of research and development. Natron Energy (formerly Alveo Energy), a battery-technology company based in Santa Clara, California, developed an unconventional anode design using a blend of elements chemically similar to the paint pigment known as Prussian blue.
According to Natron, the resulting battery has been shown to deliver up to 90 percent of its total energy in a very fast, five-minute discharge and to retain about 95 percent of its discharge capacity for 1,000 cycles. In addition, the battery is also very stable, its materials are abundant, its overall cost is competitive with conventional lead-acid batteries, and it has a lesser environmental footprint than conventional batteries. It outperforms the Department of Energy’s cycle-life and price targets for grid-scale energy storage. Just how the battery achieves its high performance, though, had puzzled the researchers.
Monovalent manganese
Typically, Li-ion and Na-ion batteries use carbon-based anodes. But in Natron’s case, both anode and cathode utilized transition metals—manganese and iron, respectively—which can exhibit various charged states.
There was speculation, dating back to a 1928 German-language journal article, that manganese could exist in a monovalent state (Mn1+), losing a single electron. This is unusual, as manganese atoms typically are known to give up two or more electrons, or no electrons, in chemical reactions. Such a novel chemical state would enable a voltage range useful for battery anodes. But there hadn’t been any measurements confirming this monovalent form of manganese.
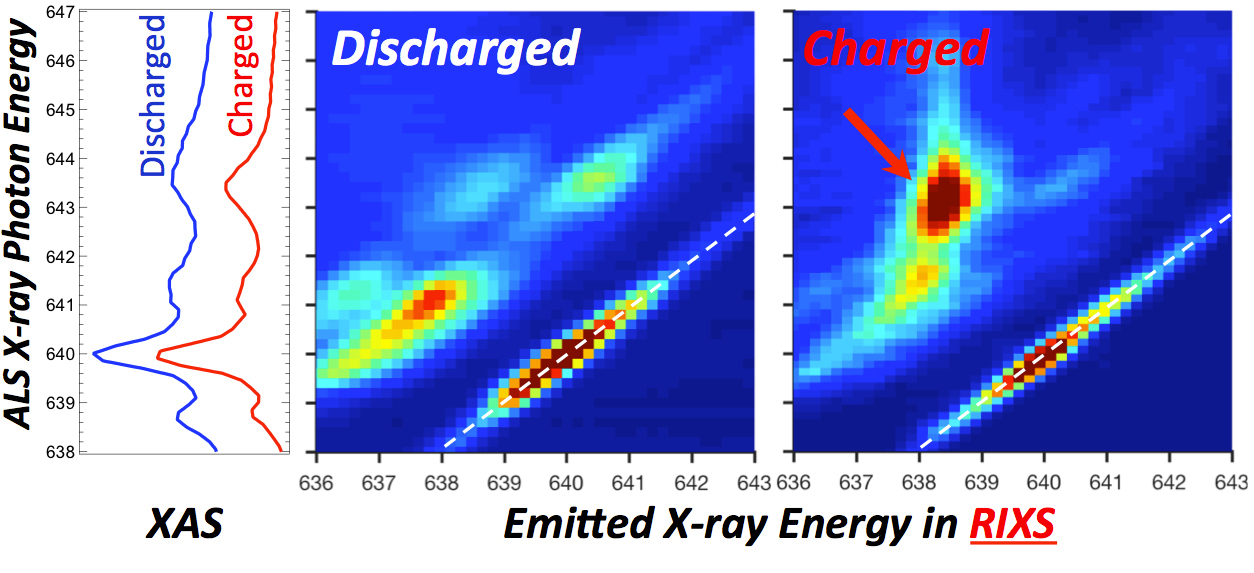
iRIXS does the trick
After initial characterization of sample battery cells at Berkeley Lab’s Molecular Foundry, the Natron team brought the cells to ALS Beamline 8.0.1. Soft x-ray absorption spectroscopy (XAS) experiments done there appeared to show mainly Mn2+, but the hint of another form led them to rely heavily on theory to speculate about a different state, with calculations performed at New York University.
The team then turned to in situ resonant inelastic x-ray scattering (iRIXS), a high-sensitivity probe of the internal chemistry of materials, also at Beamline 8.0.1. The telltale fingerprint of Mn1+, associated with carbon-coordinated Mn, appeared during the battery’s charge cycle. It turns out that Mn1+ behaves very similarly to Mn2+ in conventional spectroscopies, which is why it was difficult to detect. The results also show that the special circumstances giving rise to this state make it easier for electrons to travel in the material, explaining why the unusual electrode performs so well.
Natron has begun beta testing battery prototypes based on this work. More broadly, the results could inspire new avenues of exploration for novel battery materials.
Contacts: Wanli Yang, Colin Wessells
Researchers: A. Firouzi, S. Motallebi, C.W. Valencia, H.S. Israel, M. Fujimoto, and C.D. Wessells [(Natron Energy (formerly Alveo Energy)]; R. Qiao, Y.-D. Chuang, and W. Yang (ALS); and L.A. Wray (New York University).
Funding: U.S. Department of Energy (DOE) Advanced Research Projects Agency-Energy (ARPA-E), National Science Foundation, and Laboratory Directed Research and Development program at Berkeley Lab. Operation of the ALS is supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences Program (DOE BES).
Publication: A. Firouzi, R. Qiao, S. Motallebi, C.W. Valencia, H.S. Israel, M. Fujimoto, L.A. Wray, Y.-D. Chuang, W. Yang, and C.D. Wessells, “Monovalent manganese based anodes and co-solvent electrolyte for stable low-cost high-rate sodium-ion batteries,” Nat. Commun. 9, 861 (2018), doi:10.1038/s41467-018-03257-1.
Adapted from the Berkeley Lab press release, “Scientists Confirm Century-Old Speculation on the Chemistry of a High-Performance Battery.”
ALS SCIENCE HIGHLIGHT #373