A noncanonical amino-acid (NCAA) complex has been found to drive the self-assembly of a computationally designed protein. NCAAs are “noncanonical” because they’re not among the 20 amino acids that occur naturally in proteins. The NCAA of interest here, a compound of bipyridine and alanine (Bpy-ala), not only drives the self-assembly of designed proteins in highly specific geometries, it also has useful photochemical properties and can be modified to allow for possible use in a wide range of photophysical applications.
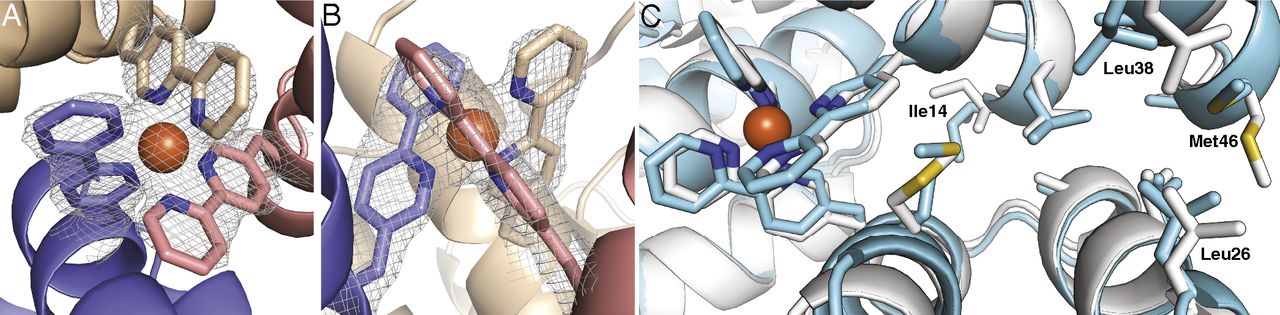
Bpy-ala forms what’s called a chelate complex, in which a metal ion, such as iron (Fe), is located at the center of a claw-like structure (“chelate” derives from the Greek word for “claw”). In the case of Bpy-ala, the claw is threefold-symmetric, and three identical protein subunits can symmetrically dock to a central [Fe(Bpy-ala)3]2+ complex.
A team led by David Baker (University of Washington) designed seven proteins, one of which proved to be stable at high concentrations. It was subjected to x-ray crystallographic analysis at ALS Beamline 8.2.1 (part of the Berkeley Center for Structural Biology) by scientists from Berkeley Lab’s Molecular Biophysics & Integrated Bioimaging Division. The results showed that the design process had near-atomic-level accuracy. Although Bpy-ala had been utilized in structural or functional capacities before, its use to drive or stabilize the formation of a protein complex had not been explored until now. In the future, these methods could be used to develop new therapeutics, biomaterials, and metalloproteins with useful optical or photochemical properties.
Work performed at ALS Beamline 8.2.1.
J.H. Mills, W. Sheffle, M.E. Ener, P.J. Almhjell, G. Oberdorfer, J.H. Pereira, F. Parmeggiani, B.Sankaran, P.H. Zwart, and D. Baker, “Computational design of a homotrimeric metalloprotein with a trisbipyridyl core,” PNAS 113, 15012 (2016). 10.1073/pnas.1600188113