RNA, like protein, can sometimes function as an enzyme (ribozyme) to speed biochemical reaction rates. But how does RNA, a simple polymer with just four different chemical building blocks, enhance reaction rates by at least a million fold? Recently, a group from the University of California, Berkeley, and the Howard Hughes Medical Institute obtained high-resolution x-ray crystallographic structures of a ribozyme trapped in different states of its catalytic cycle, showing how a change in the RNA conformation governs the reaction mechanism. Most ribozymes catalyze the cutting and pasting of RNA molecules at specific sites, snipping out (cleaving) extraneous sequences not needed in the final functional form of an RNA. In the hepatitis delta virus (HDV), a human pathogen with a small circular RNA genome, a ribozyme contained within the viral sequence cuts the RNA at a single site during replication to enable packaging of new virions, the extracellular virus particles that allow the virus to infect a host and replicate. Understanding how the ribozyme works is of interest both for defining the fundamental principles of RNA-catalyzed reactions and for discovering a possible Achilles’ heel in this deadly pathogen.
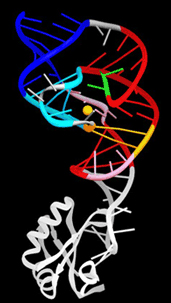
The hepatitis delta virus is so small that it cannot replicate on its own, needing hepatitis B for the process and therefore only infecting patients who already have that form of the disease. Unfortunately, super-infection with the delta virus causes a severe, fulminant form of hepatitis that is often lethal. In its replication cycle, HDV resembles small infectious RNAs called viroids, covalently closed circular RNA molecules that are found in plants. In both the delta virus and plant viroids, self-cleaving ribozymes encoded within the viral genome slice the RNA copies into unit-length pieces for viral packaging. The delta virus ribozyme is unique, however, in that once the RNA is cleaved, the ribozyme is somehow inactivated, “switching off” and preventing further RNA cleavage or re-ligation of the separated RNA strands.
To comprehend how such inactivation might occur and to determine the chemical mechanism by which the delta virus ribozyme does its work, the researchers used x-ray crystallography at the ALS to examine ten molecular structures of ribozymes trapped in the precleaved state. The catalytic site in the precursor ribozyme contains a hydrated divalent magnesium ion, which is adjacent to the phosphorus–oxygen bond to be broken. A cytidine base that is part of the ribozyme sequence is positioned in the active site on the other side of the labile phosphorus–oxygen bond. Together with supporting biochemical data, these structures suggest that the cytidine from the ribozyme activates the RNA for self-cleavage, while a magnesium-bound water molecule stabilizes the RNA as the bond is broken.
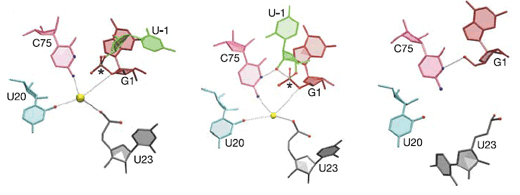
Comparison of these trapped precursor structures to each other and to the structure of the “product”—a (previously determined) self-cleaved form of the delta ribozyme—shows that the RNA undergoes a significant conformational change in the region where the cleavage reaction occurs. This structural change destroys the binding site for the catalytic magnesium ion and also moves the critical cytidine base beyond striking distance of the reactive groups in the RNA, explaining the inactivation of the ribozyme.
This unique quality of the HDV ribozyme to slice specific RNA molecules and then inactivate itself provides a mechanistic explanation for its activity during the HDV viral life cycle, information that could be useful in developing antiviral therapies that target the ribozyme.
Contact: Jennifer Doudna
Research conducted by A. Ke, F. Ding, and J.H. Cate (Univ. of California, Berkeley); K. Zhou (Howard Hughes Medical Institute); and J.A. Doudna (Univ. of California, Berkeley, Berkeley Lab, and Howard Hughes Medical Institute).
Research funding: The National Institutes of Health and the Howard Hughes Medical Institute. Operation of the ALS is supported by the U.S. Department of Energy, Office of Basic Energy Sciences.
Publication about this research: A. Ke, K. Zhou, F. Ding, J.H. Cate, and J.A. Doudna, “A conformational switch controls hepatitis delta virus ribozyme catalysis,” Nature 429, 201 (2004).
ALS SCIENCE HIGHLIGHT #86