SCIENTIFIC ACHIEVEMENT
Using the Advanced Light Source (ALS), researchers found that a symbiont capable of fixing nitrogen (turning it into a biologically usable form) has evolved into an organelle—an intrinsic part of the algae cells that host it.
SIGNIFICANCE AND IMPACT
The discovery is of great interest for understanding organelle genesis and for efforts to engineer agricultural plants with built-in nitrogen-fixing capabilities.
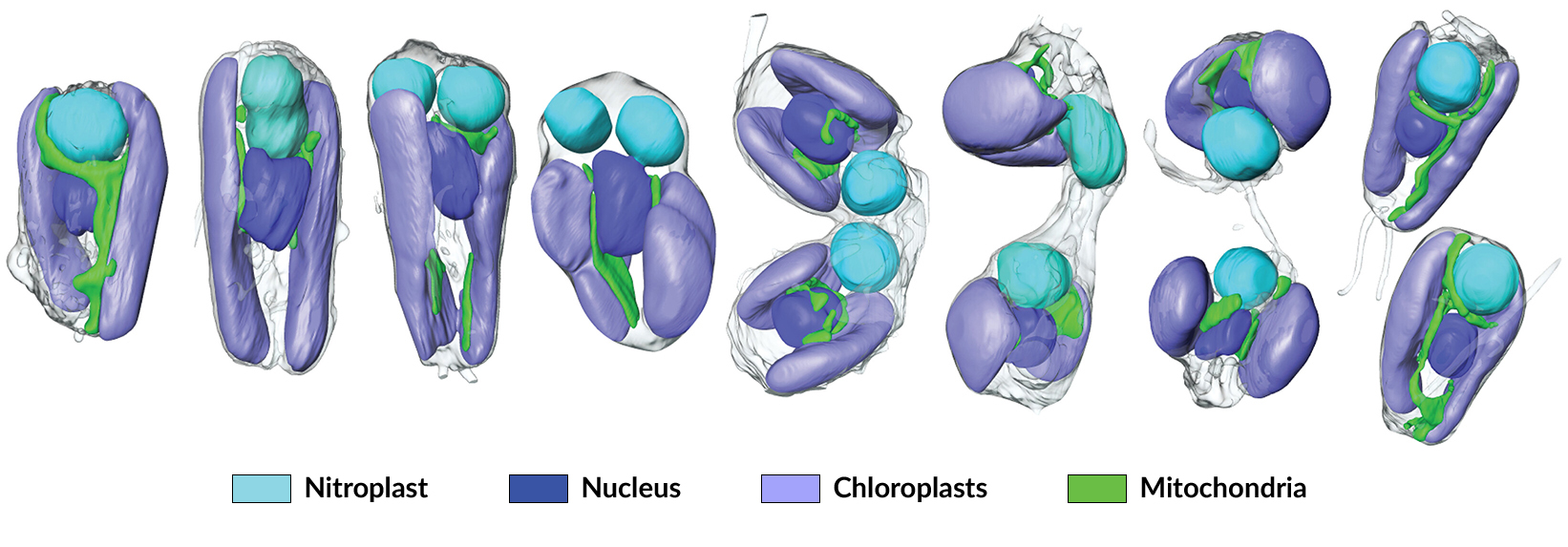
Introducing the nitroplast
Modern biology textbooks assert that only bacteria can take nitrogen from the atmosphere and convert it into a form that is usable for life. Plants that fix nitrogen, such as legumes, do so by harboring symbiotic bacteria in root nodules. But a recent discovery upends that rule.
An international team of scientists used soft x-ray tomography and proteomics (analysis of the proteins produced by an organism) to establish the first known example of a nitrogen-fixing organelle, intrinsic to certain algae cells. Dubbed “nitroplasts,” the organelles are the result of primary endosymbiosis—the process by which a symbiont is engulfed by a host organism and evolves beyond symbiosis to become part of the host cells, analogous to what occurred with mitochondria and chloroplasts.
The discovery raises the prospect of engineering plants that can fix nitrogen directly from the air, reducing the need for synthetic nitrogen fertilizers, the production of which generates enormous amounts of carbon dioxide.
A decades-long mystery
In 1998, Jonathan Zehr, a UC Santa Cruz distinguished professor of marine sciences, found a short DNA sequence that appeared to be from an unknown nitrogen-fixing cyanobacterium in Pacific Ocean seawater. Zehr and colleagues dubbed the mystery organism UCYN-A.
For years, scientists considered UCYN-A an endosymbiont—a separate organism living symbiotically inside a larger organism. But Zehr and colleagues recently showed that the size ratio between UCYN-A and their algal hosts is similar across different species of the marine algae Braarudosphaera bigelowii. The researchers used a model to demonstrate that the metabolisms of the host cell and UCYN-A are linked, controlled by the exchange of nutrients. This synchronization in growth rates led the researchers to call UCYN-A “organelle-like.” But in order to confidently call UCYN-A an organelle, the researchers needed additional lines of evidence.
In this latest work, the group showed that UCYN-A relies on proteins from its host algal cells to function and that its replication and division is tightly paired with that of the host cell, two key criteria that define an organelle.
Tomographic and proteomic evidence
At ALS Beamline 2.1, the researchers used soft x-ray tomography (SXT) to rapidly visualize internal components of B. bigelowii cells over the life cycle of the system, providing quantitative information on organelle density and composition. The data revealed close coordination between the replication and division of UCYN-A and other B. bigelowii organelles, implying control over UCYN-A division by its host, ensuring that the nitroplast gets passed down to daughter cells.
In addition, proteomics revealed that around half of the proteins needed by UCYN-A are made by the algal host cell, then labeled with a specific amino acid sequence, which tells the cell to send them to the nitroplast. The nitroplast then imports the proteins and uses them. This dependent relationship, taken together with the images of synchronized division, shows that UCYN-A deserves organelle status.
Plenty of questions about UCYN-A and its algal host remain unanswered, and scientists will likely find other organisms with evolutionary stories similar to UCYN-A. But as the first of its kind, this discovery is one for the textbooks.

Contact: Carolyn Larabell
Researchers: T.H. Coale, K.A. Turk-Kubo, W.K.E. Mak, and J.P. Zehr (UC Santa Cruz); V. Loconte, B. Vanslembrouck, J.-H. Chen, M. Le Gros, and C. Larabell (UC San Francisco, Berkeley Lab); S. Cheung (National Taiwan Ocean University); A. Ekman (UC San Francisco); K. Hagino, Y. Takano, and M. Adachi (Kochi University, Japan); and T. Nishimura (Fisheries Technology Institute, Japan).
Funding: Simons Foundation; National Institutes of Health; US Department of Energy (DOE), Office of Science, Biological and Environmental Research program; and Japan Society for the Promotion of Science. Operation of the ALS is supported by DOE Office of Science, Basic Energy Sciences program.
Publication: T.H. Coale, V. Loconte, K.A. Turk-Kubo, B. Vanslembrouck, W.K.E. Mak, S. Cheung, A. Ekman, J.-H. Chen, K. Hagino, Y. Takano, T. Nishimura, M. Adachi, M. Le Gros, C. Larabell, and J.P. Zehr, “Nitrogen-fixing organelle in a marine alga,” Science 384, 217 (2024), doi:10.1126/science.adk1075.
Adapted from the Berkeley Lab and UC Santa Cruz press releases, “Scientists Discover First Nitrogen-Fixing Organelle.”
ALS SCIENCE HIGHLIGHT #502