Type 2 diabetes mellitus (T2DM), characterized by abnormally high blood glucose levels, affects hundreds of millions of people worldwide. In the pursuit to better treat this disease, the human receptor protein GPR40 has been identified by pharmaceutical company Takeda as a potential new drug target. To this end, TAK-875 (fasiglifam), a partial agonist of GPR40, was brought into clinical development by Takeda as a possible new treatment for T2DM.
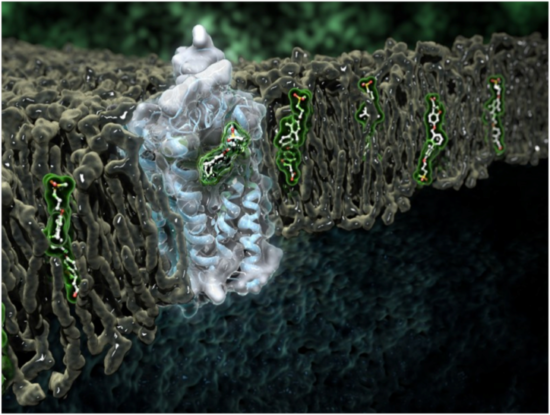
GPR40 is a signaling protein—also known as a G-protein-coupled receptor (GPCR) and it is located in the cell membrane, inside the lipid bilayer. GPR40 is a fatty-acid receptor that enhances glucose-dependent insulin secretion upon ligand binding. GPCRs are involved in many diseases and are the target of approximately 30 percent of marketed drugs. However, GPCRs are notoriously difficult to crystallize because they are integral membrane proteins.
To better understand the mode of action of TAK-875, the three-dimensional structure of GPR40 bound to TAK-875 was determined after overcoming a number of challenging experimental hurdles. The GPR40 crystals were small —about 20-30 microns—and were grown in a media that makes them barely visible to optical microscopes. So the first step before diffraction data could be collected was simply finding the tiny, almost invisible crystals. In collaboration with the Berkeley Center for Structural Biology (BCSB), which operates the ALS sector 5 protein crystallography beamlines, Takeda scientists implemented raster screening, a new technique that uses highly collimated x-rays to locate the crystals within the media.
Once the tools were in place to locate and center the small crystals, it was possible to collect a full dataset on a single crystal at Beamline 5.0.3 and to determine for the first time the novel structure of the GPR40—TAK-875 complex. Using the ALS data in combination with data collected on a dozen additional crystals at the ID23 beamlines of the Advanced Photon Source, the final structure of the complex was determined at 2.3 Ångstrom resolution and published in Nature.
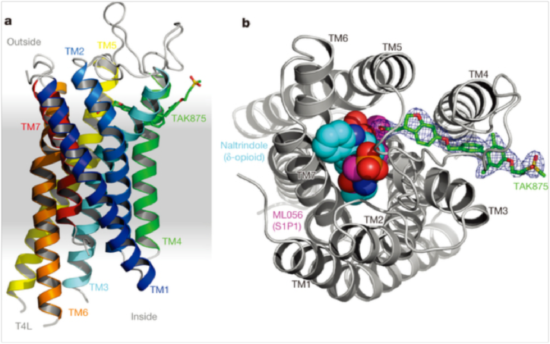
The structure provided surprising insights into how the receptor and the drug interact with each other. TAK-875 binds in a channel flanked by transmembrane helices 3 and 4, and not in the ligand binding site usually observed in other GPCR structures (called the orthosteric binding site). “It appears that the drug enters the protein through the lipid bilayer, which we didn’t expect,” says Gyorgy Snell, a co-author on the Nature paper and Takeda scientist working at the ALS. “What made it most interesting was that this was the first time that anyone was able to actually see this new ligand binding location.”
Takeda California (formerly Syrrx, Inc.) has been using the ALS since 2002, and is one of the original Participating Research Team (PRT) members of the Berkeley Center for Structural Biology (BCSB). The high degree of performance and automation at Beamlines 5.0.3 and 5.0.2 have helped Takeda California scientists to successfully advance several structure-based drug discovery projects to clinical evaluation, says Snell.
Publication about this research: Srivastava A, Yano J, Hirozane Y, Kefala G, Gruswitz F, Snell G, Lane W, Ivetac A, Aertgeerts K, Nguyen J, Jennings A, Okada K, “High-resolution structure of the human GPR40 receptor bound to allosteric agonist TAK-875.” Nature 513, 124-7 (2013).