SCIENTIFIC ACHIEVEMENT
Researchers determined the structure of a human antibody that broadly protects against a bacterium that causes meningitis and sepsis.
SIGNIFICANCE AND IMPACT
The work provides molecular-level information about how the antibody confers broad immunity against a variable target and suggests strategies for further improvement of available vaccines.
Meningococcal disease
Our central nervous systems (brain and spinal cord) are surrounded by three membranes called “meninges.” Meningitis is caused by the swelling of these membranes, resulting in headache, fever, and neck stiffness. Most cases of meningitis in the United States are the result of viral infections and are relatively mild. However, meningitis caused by bacterial infection, if left untreated, can be deadly or lead to serious complications, including hearing loss and neurologic damage.
The bacterium responsible for meningitis (Neisseria meningitidis) can also infect the bloodstream, causing another life-threatening condition known as sepsis. N. meningitidis is spread through close contact (coughing or kissing) or lengthy contact (e.g. in dorm rooms or military barracks). In this work, researchers were interested in understanding how humans develop immunity to bacterial meningitis and sepsis, collectively known as meningococcal disease, by vaccination with a new protein-based vaccine.
Vaccines for serogroup B
N. meningitidis bacteria can be divided into several types, or serogroups, based on whether or not they share common antigens (the molecules targeted by antibodies). Five serogroups (designated A, B, C, W, and Y) are responsible for most cases of meningococcal disease. Vaccines for serogroups A, C, W, and Y have been available for some time, but vaccines for serogroup B proved to be more difficult to develop.
In 2014, two vaccines for serogroup B were approved for use in the United States: 4CMenB (trade name Bexsero, from GlaxoSmithKline) and Bivalent rLP2086 (trade name Trumenba, from Pfizer). A key component of both vaccines is an antigen called factor H binding protein (fHbp), a surface-exposed meningococcal lipoprotein that induces high levels of bactericidal antibodies.
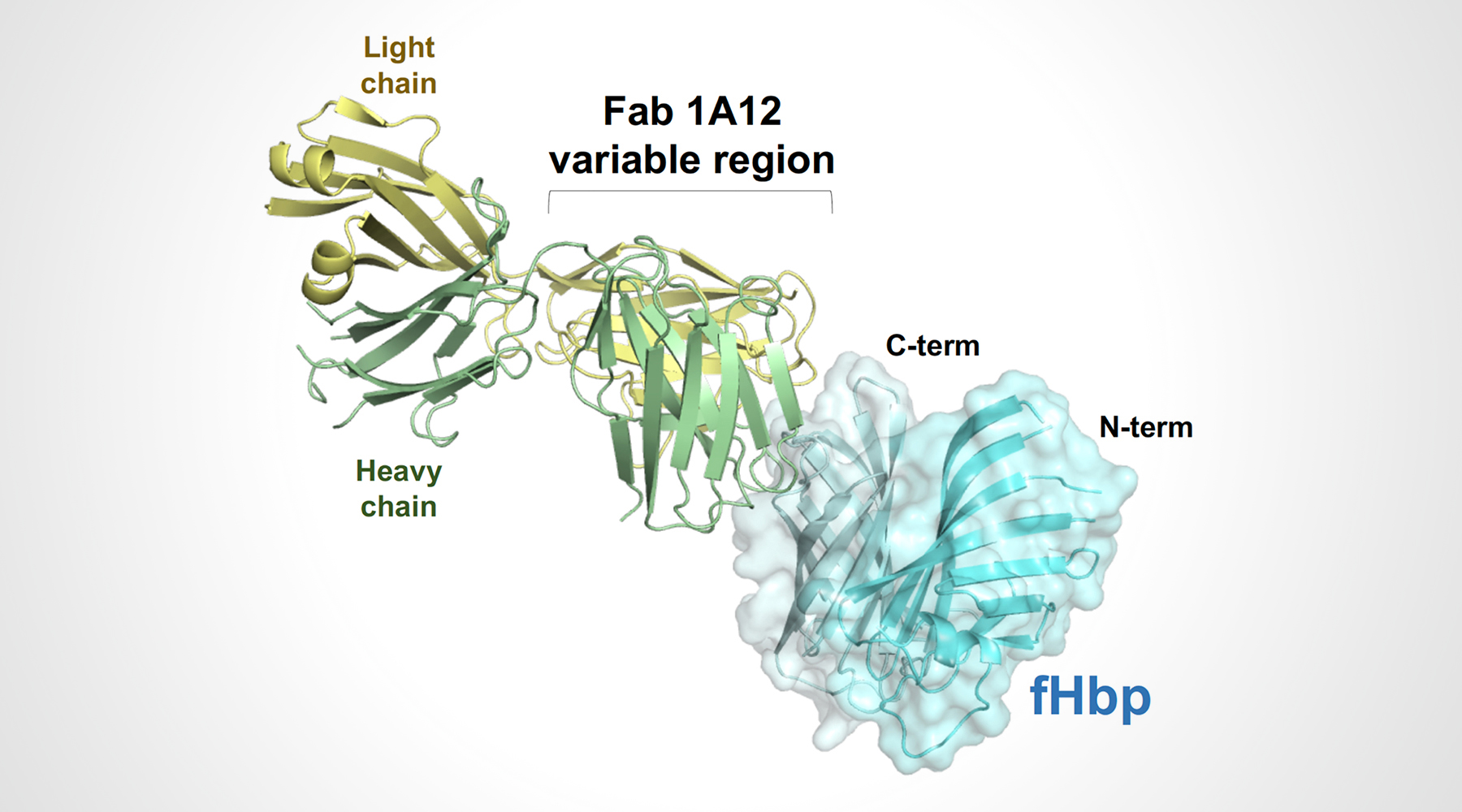
In a previous study, the researchers isolated 10 anti-fHbp antibody fragments (Fabs) from human subjects vaccinated with 4CMenB. One of the antibody fragments (Fab 1A12) was found to cross-react with several fHbp variants that had different amino-acid sequences, including representatives from all three naturally occurring fHbp variant groups (variants 1.1, 2.16, and 3.45). This was unusual because most known antibodies against fHbp are specific to only one of the variant groups.
Crystallographic analysis
To understand the structural basis of these broad antigen-recognition properties, the researchers crystallized Fab 1A12 alone and in complex with fHbp. High-resolution protein crystallography was performed at ALS Beamline 5.0.1, part of the Berkeley Center for Structural Biology (BCSB), and at the European Synchrotron Radiation Facility (ESRF).
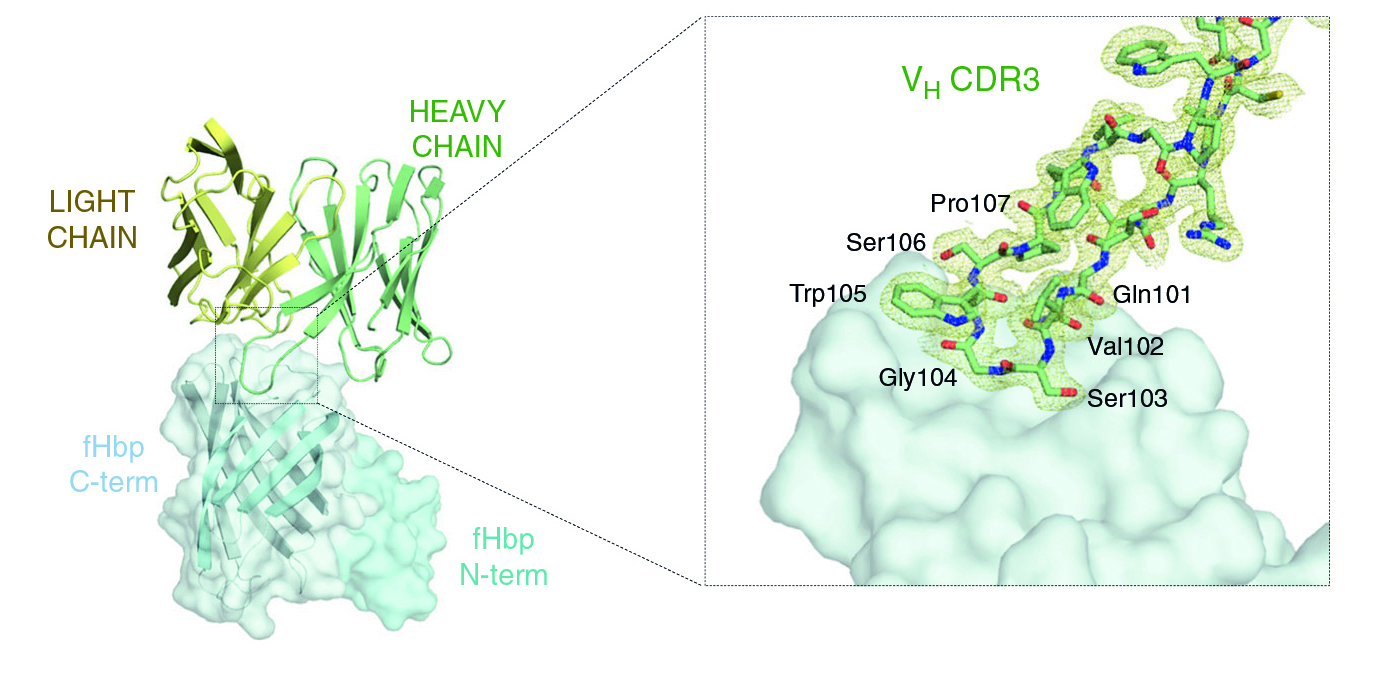
The structure of the antigen–antibody complex showed that Fab 1A12 targets an area of the antigen that is highly conserved across the three fHbp variants. A close inspection of the Fab 1A12/fHbp interface revealed a predominant role in antigen recognition for one Fab subunit (the heavy chain), and especially for one loop that extends into a notable groove on the fHbp surface. In addition, the free Fab structure highlighted conformational rearrangements that occur upon antigen binding.
Finally, measurements of bactericidal activity showed that antibody 1A12 is not only cross-reactive, but also cross-protective, killing meningococci in all three of the fHbp variant groups. The work shows in principle that a variable protein can elicit cross-protective antibodies and suggests strategies for further improvement of the vaccine.

Contact: Peter Beernink
Researchers: J. López-Sagaseta, F. Bianchi, L. Santini, E. Frigimelica, and M. Pizza (GSK Vaccines, Siena, Italy); P.T. Beernink and A.H. Lucas (UCSF Benioff Children’s Hospital, Oakland, CA); and M.J. Bottomley (GSK Vaccines, Rockville, MD).
Funding: GSK Vaccines (Italy), European Commission Horizon 2020 Program, University of Florence (Italy), and National Institutes of Health. Operation of the ALS is supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences Program (DOE BES).
Publication: J. López-Sagaseta, P.T. Beernink, F. Bianchi, L. Santini, E. Frigimelica, A.H. Lucas, M. Pizza, and M.J. Bottomley, “Crystal structure reveals vaccine elicited bactericidal human antibody targeting a conserved epitope on meningococcal fHbp,” Nat. Commun. 9, 528 (2018), doi:10.1038/s41467-018-02827-7.
ALS SCIENCE HIGHLIGHT #384