SCIENTIFIC ACHIEVEMENT
Using specialized equipment at the Advanced Light Source (ALS), including a custom-built reaction cell, researchers uncovered the role of manganese in cobalt manganese oxide catalysts used for fuel production.
SIGNIFICANCE AND IMPACT
This work opens the door to improved catalyst designs that could decrease the production of harmful methane byproducts in a common petrochemical process.
Sustainable fuel production
First developed in the 1920s, the Fischer-Tropsch synthesis remains a common chemical process used to convert carbon monoxide and hydrogen from coal into liquid hydrocarbons, or fuel. Cobalt is an efficient catalyst for this reaction, and its combination with manganese has been known for decades to further improve the process by promoting the preferential production of long-chain hydrocarbons over methane, a contributor to climate change. However, the molecular-scale origin for why manganese improves the efficiency of this reaction remains unclear.
In this work, researchers uncovered the role of manganese in cobalt manganese oxide systems by combining well-defined model catalysts with advanced x-ray spectroscopy techniques. These results provide a platform for how customized equipment can answer challenging scientific questions and set the stage for new catalyst designs that may further decrease the production of methane during Fischer-Tropsch synthesis.
Custom-built instrumentation
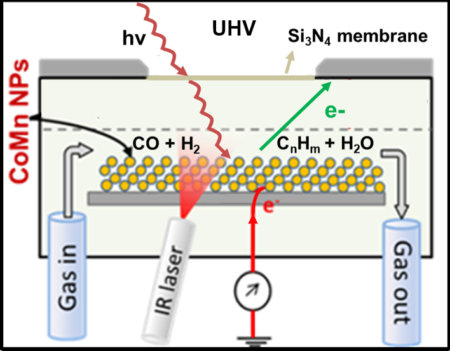
Numerous studies have investigated the mechanisms for catalytic performance in cobalt manganese oxide systems, proposing particular interfaces, mixed oxides, or nanostructures as reasons for the improved efficiency. However, due to the heterogeneity of widely used powder catalysts, resulting in separated domains of cobalt and manganese, the molecular-scale mechanism of these catalysts remains under debate. To circumvent this, the researchers created model catalysts of well-defined cobalt manganese oxide nanocrystals and films where the components were intermixed at the sub-nanometer scale.
The model catalysts were investigated using ambient-pressure x-ray photoelectron spectroscopy (APXPS) at Beamline 9.3.2, which is equipped with commercial instrumentation uniquely designed for ambient-condition experiments that mimic real reaction conditions (this instrumentation was previously developed by the researchers, is now available at Beamline 9.0.2 and Beamline 11.0.2.1, and induced the application of APXPS at other synchrotron facilities). Similarly, to achieve realistic reaction conditions for x-ray absorption spectroscopy (XAS), a custom-built reaction cell was designed for Beamline 8.0.1, allowing experiments that typically occur under high vacuum to be performed under ambient pressure. The challenging and iterative process of perfecting this reaction cell was key to the success of this study, and the reaction cell is now available to other ALS users.
The magic of manganese
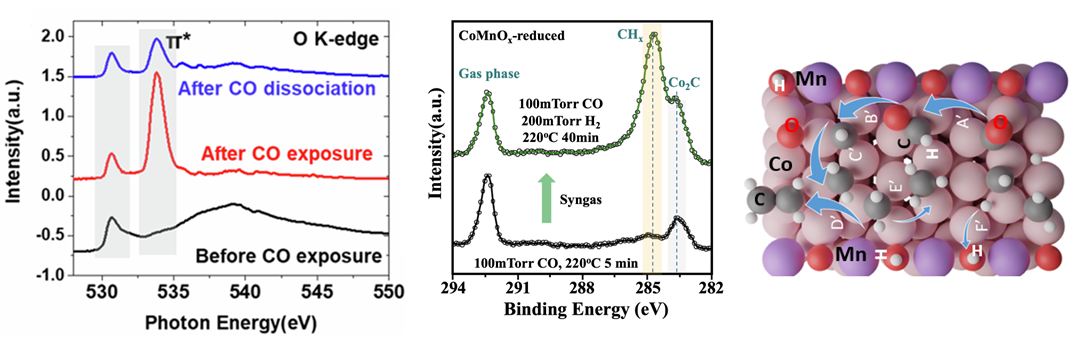
Using the custom-built reaction cell for XAS, the researchers were able to observe the real-time breakdown of carbon monoxide during the introduction of hydrogen to the cobalt manganese oxide catalyst at ambient conditions. Next, APXPS showed a significant increase in CHx hydrocarbon species after the addition of carbon monoxide and hydrogen on the cobalt manganese oxide catalyst surface—which was in stark contrast to the systems without manganese, where the production of cobalt carbide was more dominant instead. In other words, these results demonstrated that the addition of manganese creates more CHx, which ultimately allows for the production of more long-chain hydrocarbons.
The ALS data was complemented by computational density functional theory (DFT) calculations. DFT demonstrated that manganese helps with the production of long-chain hydrocarbons because manganese oxide binds with hydrogen, making it unavailable for reacting with CHx to stop propagation, resulting in less methane and more long-chain hydrocarbons. Moving forward, this work paves the way for improved catalyst designs that can make these reactions even more efficient.
Contacts: Hao Chen and Miquel Salmeron
Researchers: H. Chen, X. Zhao, J. Oliver-Meseguer, J. Yang, E. Pach, S. Carenco, L. Treps, N. Liakakos, V. Altoe, E. Wong, and J. Su (Berkeley Lab); Z. Lian and N. López (Barcelona Institute of Science and Technology); J. Wan, Y. Shan, H. Zheng, P. Yang, A.T. Bell, and M. Salmeron (Berkeley Lab and UC Berkeley); P.F. Pieters (UC Berkeley); Z. Zhuo, F. Yang, J. Guo, and M. Blum (ALS); S.H. Lapidus (Argonne National Laboratory); A. Hunt and I. Waluyo (Brookhaven National Laboratory); and H. Ogasawara (SLAC National Accelerator Laboratory).
Funding: US Department of Energy, Office of Science, Basic Energy Sciences program (DOE BES); National Science Foundation–US-Israel Binational Science Foundation (NSF-BSF); European Union Horizon research and innovation program; and Spanish Ministry of Science and Innovation. Operation of the ALS, NSLS-II, and SSRL is supported by DOE BES.
Publication: H. Chen, Z. Lian, X. Zhao, J. Wan, P.F. Pieters, J. Oliver-Meseguer, J. Yang, E. Pach, S. Carenco, L. Treps, N. Liakakos, Y. Shan, V. Altoe, E. Wong, Z. Zhuo, F. Yang, J. Su, J. Guo, M. Blum, S.H. Lapidus, A. Hunt, I. Waluyo, H. Ogasawara, H. Zheng, P. Yang, A.T. Bell, N. López, and M. Salmeron, “The role of manganese in CoMnOx catalysts for selective long-chain hydrocarbon production via Fischer-Tropsch synthesis,” Nat. Commun. 15, 10294 (2024), doi:10.1038/s41467-024-54578-3.
ALS SCIENCE HIGHLIGHT #515