SCIENTIFIC ACHIEVEMENT
With the help of the Advanced Light Source (ALS), researchers from UC Berkeley and ExxonMobil fine-tuned a material to capture CO2 in the presence of water.
SIGNIFICANCE AND IMPACT
The parties have applied for a patent on the material, which was developed for use on the relatively humid flue gases emitted by certain natural gas power plants, a cleaner-burning alternative to coal.
The power of natural gas
About 65% of anthropogenic greenhouse gas emissions comes from the combustion of fossil fuels in power plants. So far, efforts to capture CO2 from power-plant flue gases and sequester it underground have mainly focused on coal-fired power plants. However, in the United States, natural gas has surpassed coal in the amount CO2 released, despite the fact that natural gas emits approximately half as much CO2 per unit of electricity. Therefore, new materials are urgently needed to address this situation.
Not all combustion is alike
Compared to coal-fired power plants, natural gas combined cycle (NGCC) plants produce flue gases with low CO2 concentrations. This reduces the carbon footprint, but increases the technical difficulty of CO2 capture. Also, materials capable of adsorbing such low concentrations of CO2 often require high temperatures to release it for sequestration, an important part of the cycle that offsets initial low-carbon benefits. NGCC emissions also have a higher concentration of O2, which has a corrosive effect on adsorbent materials, and both NGCC and coal flue streams are saturated in water, which can both degrade materials and reduce efficiency. Thus, an effective NGCC CO2-capture material must selectively bind low-concentration CO2 under humid conditions while being thermally and oxidatively stable.
Earlier discovery and optimization
At NGCC plants, CO2 capture is currently done primarily using solutions of ammonia derivatives (amines). However, releasing the CO2 from these liquids is energy intensive and expensive. In previous work done at the Center for Gas Separations (a DOE Energy Frontier Research Center), UC Berkeley researchers discovered a metal–organic framework (MOF) that releases CO2 with much less energy. The work attracted the attention of ExxonMobil, which was interested in optimizing the new material to make it even more effective. In this work, the Berkeley group, with support from ExxonMobil, worked on fine-tuning the MOF by functionalizing it with cyclic diamine compounds.
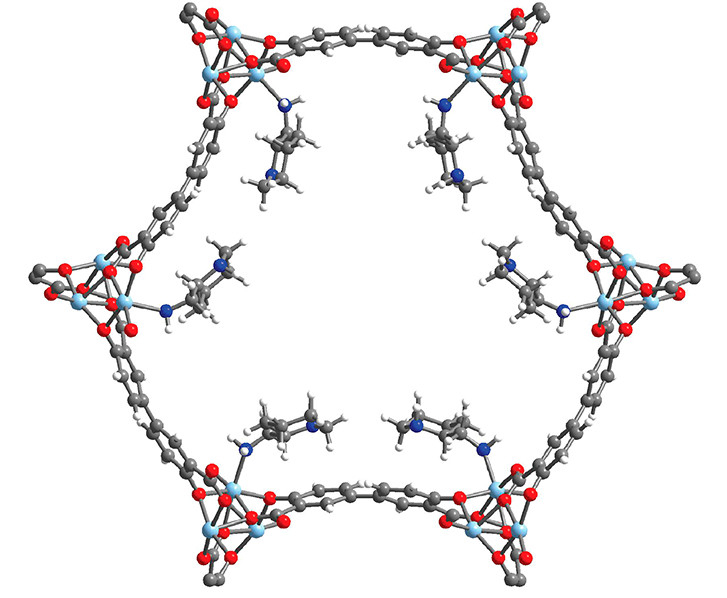
Characterization by diffraction
At ALS Beamline 12.2.1, the researchers used single-crystal x-ray diffraction to precisely determine the structure of 2-ampd–Zn2(dobpdc), a crystal with the same structure as the functionalized MOF. The information made clear how the diamines—the organic molecules that actually react with CO2—are organized. The researchers were thus able to compare the structure with predictions from computational work and recognize how water might affect CO2 adsorption. The beamline also offers the ability to dose samples with gas, an experiment the researchers are currently working on to see how CO2 actually interacts with the MOF binding sites and to further correlate structure with adsorption behavior.
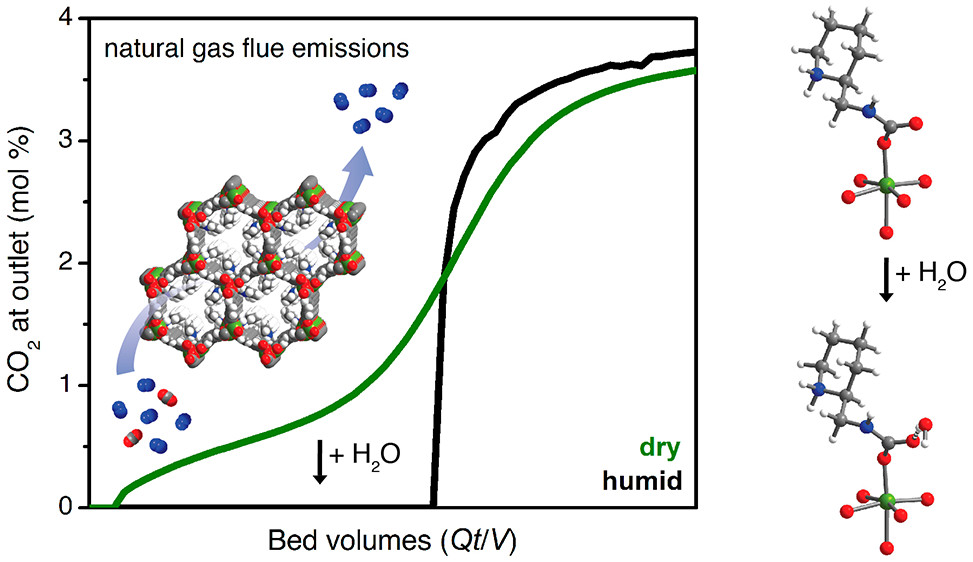
A surprising watery advantage
Adsorption measurements confirmed that 2-ampd–Mg2(dobpdc) is capable of adsorbing over 90% of the CO2 in humid natural gas emissions. The researchers also discovered that humidity actually improves the material’s CO2 adsorption. Based on spectroscopic studies and theoretical calculations, they concluded that certain structures that form upon CO2 binding are affected by the presence of water, making the CO2 bind more strongly while preserving the ability to cycle CO2 in and out with small temperature swings. UC Berkeley and ExxonMobil have applied for a patent on this particular MOF variant, in light of its exceptional and unique ability to capture CO2 from NGCC flue gases.
Contact: Jeffrey Long
Researchers: R.L. Siegelman, P.J. Milner, J.-H. Lee, J.A. Reimer, and J.R. Long (UC Berkeley and Berkeley Lab); A.C. Forse and K.A. Colwell (UC Berkeley); J.B. Neaton (UC Berkeley, Berkeley Lab, and Kavli Energy Nanosciences Institute); and S.C. Weston (ExxonMobil Research and Engineering Company).
Funding: ExxonMobil Research and Engineering Company, National Institutes of Health, Philomathia Foundation, Berkeley Energy and Climate Institute, and University of California, Berkeley. Operation of the ALS is supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences Program.
Publication: R.L. Siegelman, P.J. Milner, A.C. Forse, J.-H. Lee, K.A. Colwell, J.B. Neaton, J.A. Reimer, S.C. Weston, and J.R. Long, “Water Enables Efficient CO2 Capture from Natural Gas Flue Emissions in an Oxidation-Resistant Diamine-Appended Metal–Organic Framework,” J. Am. Chem. Soc. 141, 13171 (2019), doi:10.1021/jacs.9b05567.
ALS SCIENCE HIGHLIGHT #413