Rotavirus, a major cause of infantile gastroenteritis, is responsible for the deaths of about 200,000 children per year, mostly in developing countries. Although vaccines are available that reduce the severity and incidence of the illness, the the virus continues to circulate in human and animal reservoirs, and scientists continue investigations into how rotavirus molecular structures relate to function.
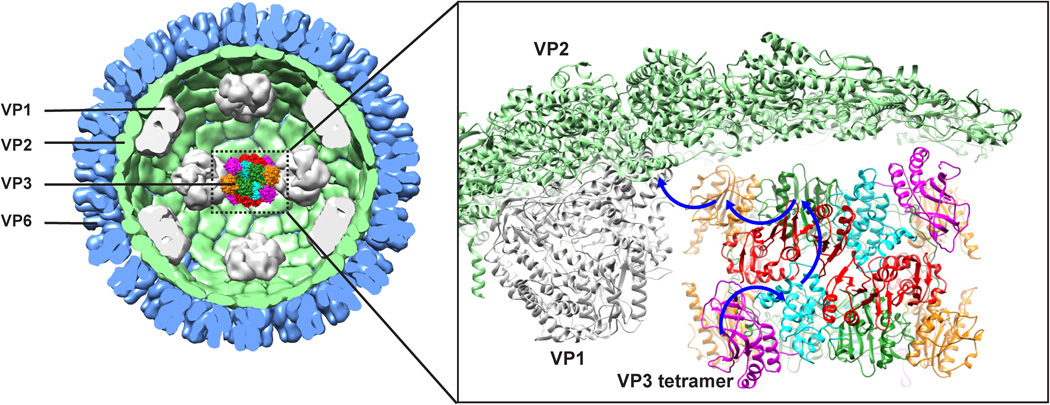
Of the six proteins that form the rotavirus particle, only one—viral protein 3 (VP3)—had a structure that remained unsolved after 30 years. Scientists had deduced that VP3 was necessary for “capping” the viral messenger RNA (mRNA), enabling it to mimic the host’s capped RNA and thus evade immune response.
The capping process involves a well-orchestrated set of enzymatic activities: separation of the nascent transcript from the template RNA by a helicase, removal of a terminal phosphate by an RTPase, addition of a guanylyl group by a GTase, and addition of two methyl groups by an MTase.
Combining cryo-electron microscopy, biochemical assays, and protein crystallography at Advanced Light Source Beamline 5.0.2 (part of the Berkeley Center for Structural Biology), researchers from the Baylor College of Medicine discovered that VP3 incorporates in one place all the enzymatic activities required to effectively cap rotavirus mRNA, making it unique among viral-capping enzymes.
The studies showed that VP3, as a tetramer, in addition to previously characterized GTase and MTase activities, exhibits novel RTPase and helicase activities through a modular domain organization. This VP3 structure, with such a confluence of enzymatic activities, provides a rational platform for designing antivirals to counter rotavirus infection.
D. Kumar, X. Yu, S.E. Crawford, R. Moreno, J. Jakana, B. Sankaran, R. Anish, S. Kaundal, L. Hu, M.K. Estes, Z. Wang, and B.V. Venkataram Prasad, “2.7 Å cryo-EM structure of rotavirus core protein VP3, a unique capping machine with a helicase activity,” Sci. Adv. 6, eaay6410 (2020), doi:10.1126/sciadv.aay6410.