SCIENTIFIC ACHIEVEMENT
Using the Advanced Light Source (ALS), researchers from Gilead Sciences Inc. solved the structure of an experimental HIV drug bound to a novel target: the capsid protein that forms a shield around the viral RNA.
SIGNIFICANCE AND IMPACT
The work could lead to a long-lasting treatment for HIV that overcomes the problem of drug resistance and avoids the need for burdensome daily pill-taking.
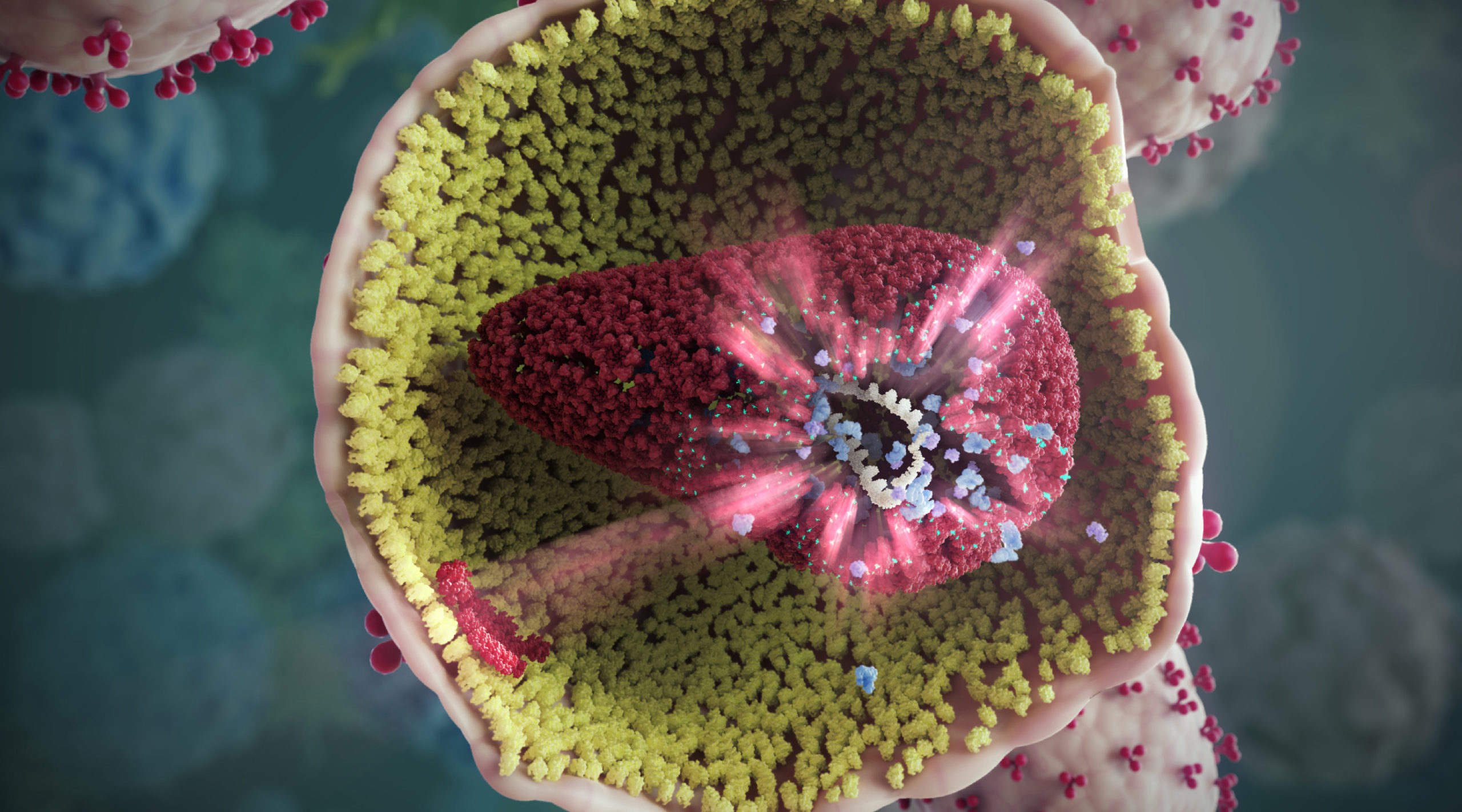
Progress in HIV treatment still needed
Over the past couple of decades, safe and effective treatment for HIV infection has turned what was once a death sentence into a chronic disease. Today, people on the latest HIV drugs have near-normal life expectancy.
However, many people are still living with multidrug-resistant HIV, unable to control their virus loads with currently available HIV drugs. The virus develops resistance when people take their pills inconsistently due to forgetfulness, side effects, or a complex schedule. To some, taking a pill every day is a burden: they schedule their lives around it for fear of missing a dose. To others, it is a stigma, as it makes it difficult to hide one’s HIV status from close friends and family.
In this work, researchers from Gilead Sciences Inc. investigated a promising small-molecule drug (GS‑6207) they developed that, if successful, could avoid issues of drug resistance while imparting longer-lasting viral suppression, enabling a more manageable regimen.
A drug with a novel mechanism of action
Most HIV drugs work by blocking viral enzymes—proteins that initiate key steps in the viral life cycle. In contrast, GS‑6207 works by targeting the HIV-1 capsid protein (CA), a structural protein that forms pinwheel-like hexamers that assemble into a conical shell (a capsid) around the viral genome. CA proteins are slow to mutate, and thus are less likely to develop resistance to GS‑6207.
Drawing on earlier x-ray protein crystallographic information that they collected at the ALS, Gilead researchers designed GS‑6207 to bind tightly between CA monomers, where it would interfere with multiple phases of the viral replication cycle. However, further studies were needed to verify that the drug molecule interacts with the CA proteins as intended.
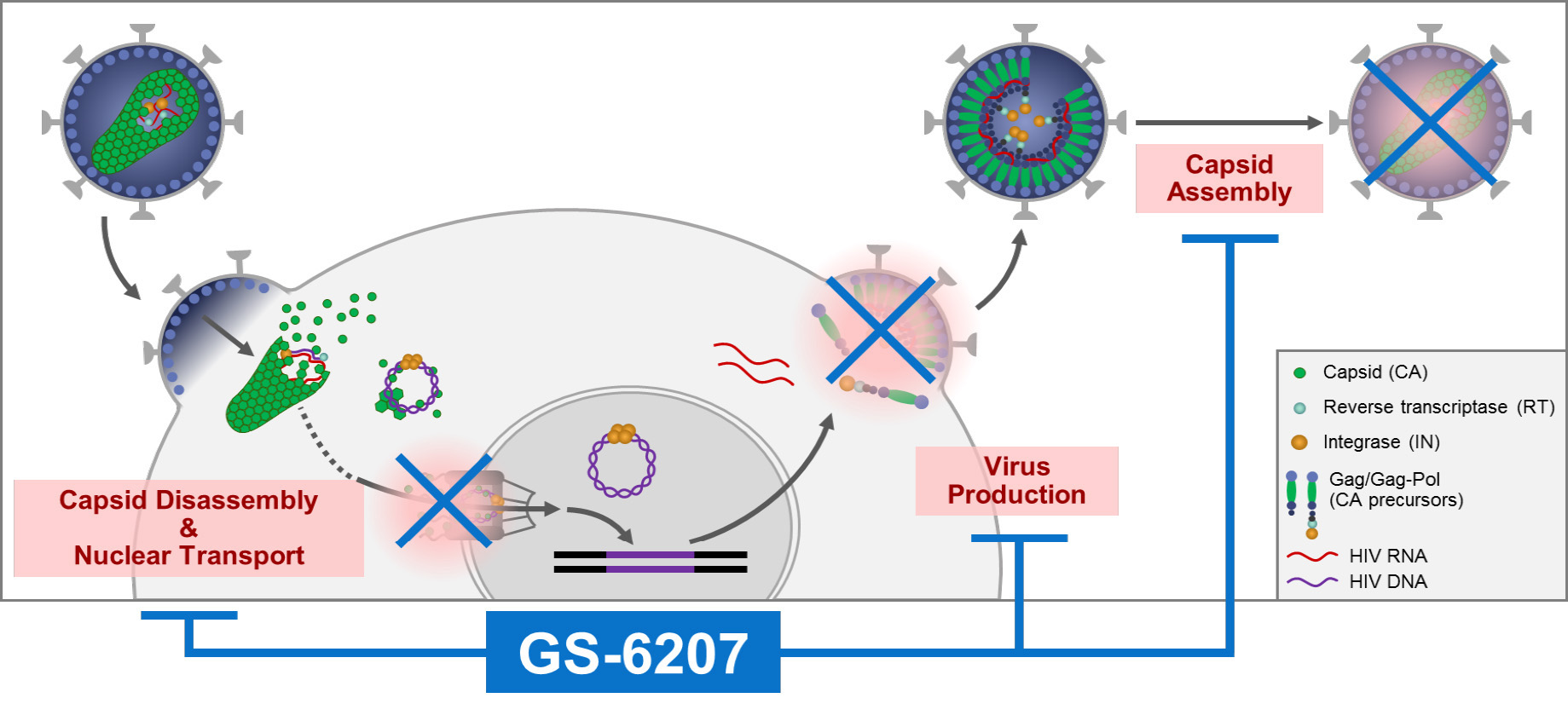
Structural studies show a snug fit
At ALS Beamline 5.0.1, the researchers performed protein crystallography experiments on single crystals of HIV CA hexamers co-crystallized with GS‑6207. The characteristics of Beamline 5.0.1 allowed for rapid data collection so that numerous samples could be screened, leading to the identification of one crystal that diffracted to a high resolution of 2.0 Å.
The resulting structure provided the molecular details of interactions that established the tight fit of GS‑6207 to HIV CA proteins, as originally designed. Despite the relatively large size of GS‑6207, the majority of the molecule was shown to be in contact with protein atoms, sandwiched between CA monomers.
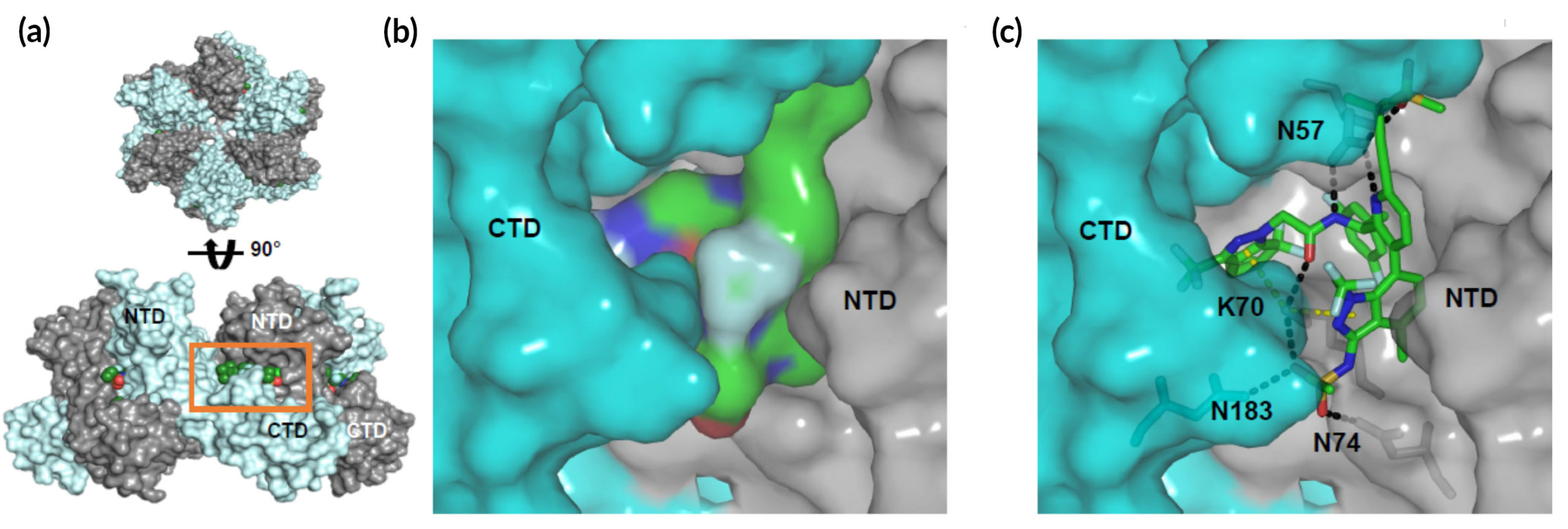
In vitro experiments and clinical trials
The molecular details of the structure supported the results from in vitro experiments, which showed a dose-dependent increase in the rate and extent of HIV capsid formation. In the context of an HIV-infected cell, this leads to malformed HIV capsids, disrupting replication.
In Phase 1 clinical trials, researchers were able to demonstrate GS‑6207’s antiviral activity in people living with HIV, validating its potential as a potent, subcutaneously injected, long-acting agent.
In future clinical trials, the researchers expect GS‑6207 to have full activity even in people with multi-class resistant HIV, and to work synergistically with other HIV drugs, without cross-resistance or cytotoxicity. As a tool for prevention, GS‑6207 has a potential to have even larger impact on public health by reducing the number of new infections.
Contact: Martin Rhee
Researchers: J.O. Link, M.S. Rhee, W.C. Tse, J. Zheng, J.R. Somoza, W. Rowe, R. Begley, A. Chiu, A. Mulato, D. Hansen, E. Singer, L.K. Tsai, R.A. Bam, C.‑H. Chou, E. Canales, G. Brizgys, J.R. Zhang, J. Li, M. Graupe, P. Morganelli, Q. Liu, Q. Wu, R.L. Halcomb, R.D. Saito, S.D. Schroeder, S.E. Lazerwith, S. Bondy, D. Jin, M. Hung, N. Novikov, X. Liu, A.G. Villaseñor, C.E. Cannizzaro, E.Y. Hu, R.L. Anderson, T.C. Appleby, B. Lu, J. Mwangi, A. Liclican, A. Niedziela‑Majka, G.A. Papalia, M.H. Wong, S.A. Leavitt, Y. Xu, D. Koditek, G.J. Stepan, H. Yu, N. Pagratis, S. Clancy, S. Ahmadyar, T.Z. Cai, S. Sellers, S.A. Wolckenhauer, J. Ling, C. Callebaut, N. Margot, R.R. Ram, Y.‑P. Liu, R. Hyland, D.M. Brainard, L. Lad, S. Swaminathan, R. Sakowicz, A.E. Chester, W.E. Lee, S.R. Yant, and T. Cihlar (Gilead Sciences, Inc.); G.I. Sinclair (AIDS Arms Inc., DBA Prism Health North Texas); P.J. Ruane (Ruane Clinical Research Group Inc.); G.E. Crofoot (The Crofoot Research Center Inc.); C.K. McDonald (Texas Centers for Infectious Disease Associates); W.I. Sundquist (University of Utah School of Medicine); and E.S. Daar (UCLA).
Funding: Gilead Sciences, Inc. Operation of the ALS is supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences program.
Publication: J.O. Link, M.S. Rhee, W.C. Tse, J. Zheng, J.R. Somoza, W. Rowe, R. Begley, A. Chiu, A. Mulato, D. Hansen, E. Singer, L.K. Tsai, R.A. Bam, C.‑H. Chou, E. Canales, G. Brizgys, J.R. Zhang, J. Li, M. Graupe, P. Morganelli, Q. Liu, Q. Wu, R.L. Halcomb, R.D. Saito, S.D. Schroeder, S.E. Lazerwith, S. Bondy, D. Jin, M. Hung, N. Novikov, X. Liu, A.G. Villaseñor, C.E. Cannizzaro, E.Y. Hu, R.L. Anderson, T.C. Appleby, B. Lu, J. Mwangi, A. Liclican, A. Niedziela‑Majka, G.A. Papalia, M.H. Wong, S.A. Leavitt, Y. Xu, D. Koditek, G.J. Stepan, H. Yu, N. Pagratis, S. Clancy, S. Ahmadyar, T.Z. Cai, S. Sellers, S.A. Wolckenhauer, J. Ling, C. Callebaut, N. Margot, R.R. Ram, Y.‑P. Liu, R. Hyland, G.I. Sinclair, P.J. Ruane, G.E. Crofoot, C.K. McDonald, D.M. Brainard, L. Lad, S. Swaminathan, W.I. Sundquist, R. Sakowicz, A.E. Chester, W.E. Lee, E.S. Daar, S.R. Yant, and T. Cihlar, “Clinical targeting of HIV capsid protein with a long-acting small molecule,” Nature 584, 614 (2020), doi: 10.1038/s41586-020-2443-1.
ALS SCIENCE HIGHLIGHT #430