SCIENTIFIC ACHIEVEMENT
Time-resolved, high-throughput, small-angle x-ray scattering (SAXS) at the Advanced Light Source (ALS) improved the screening of small-molecule drug candidates, providing insight into how they stimulate structural transitions in protein targets.
SIGNIFICANCE AND IMPACT
The work will speed the discovery of treatments designed to activate biomolecular dynamics associated with desired therapeutic outcomes.
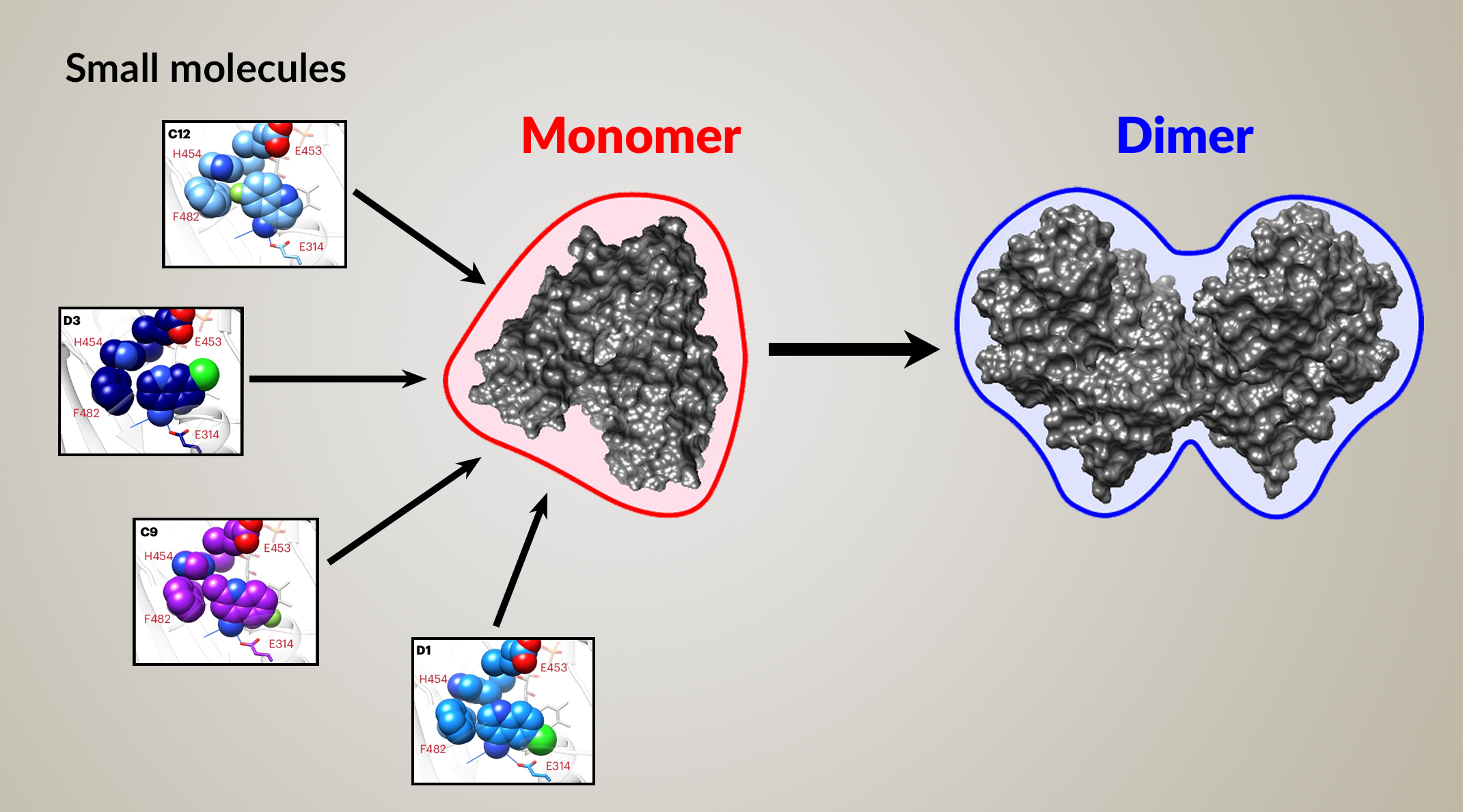
The drug-discovery pipeline
Drug discovery relies on the identification of small molecules capable of interacting with protein targets (“hits”). However, many drug-discovery workflows don’t evaluate the impact of hits upon protein form and function until later development phases. Early access to conformational information has the potential to focus hits toward mechanistically important structural states. Moreover, many targets of biological importance exist in more than one conformational state, with important biology associated with transitions between them.
Here, researchers present a discovery pipeline that integrates time-resolved, high-throughput, small-angle x-ray scattering (TR-HT-SAXS). They applied this new workflow to an important mitochondrial protein called apoptosis-inducing factor (AIF). By monitoring AIF states over time, TR-HT-SAXS can identify small molecules that modify the protein’s function, enabling researchers to target the most promising compounds in drug screens.
A mitochondrial protein pathway
AIF functions as part of a pathway for importing proteins into mitochondria. The donation of electrons to AIF (chemical reduction) precedes its dimerization, which then facilitates mitochondrial import. If AIF cannot do this efficiently, it results in symptoms associated with muscle degeneration or neurodegeneration in patients carrying certain AIF genetic mutations.
In this SAXS-augmented screening workflow, the researchers started out with a library of 2,500 compounds. They performed an initial screening that determined whether a molecule binds to AIF. They then advanced the top performers to the SAXS portion of the screening, to find out whether those molecules induce AIF dimerization.
SAXS captures proteins in transition
TR-HT-SAXS at ALS Beamline 12.3.1 (SIBYLS) can provide structural information on more than one state in a single screening experiment, provided that an appropriate conformational trigger is available. Targets that rearrange in response to oxidation-reduction processes are uniquely suited to monitoring by TR-HT-SAXS, as x-ray exposure can trigger conversion from oxidized states to x-ray-stimulated reduced states, allowing candidate molecules to interact with both.
Each small-molecule candidate was mixed with AIF in solution and repeatedly probed with x-rays. The scattering curves were then compared to each other and to reference curves for the AIF monomer and dimer. The results were summarized in SAXS similarity matrices, where the color of each cell indicates the similarity between two scattering curves.
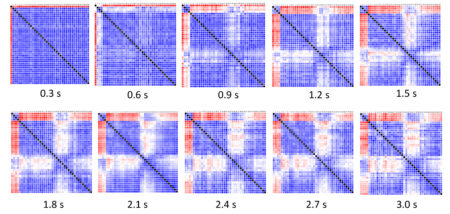
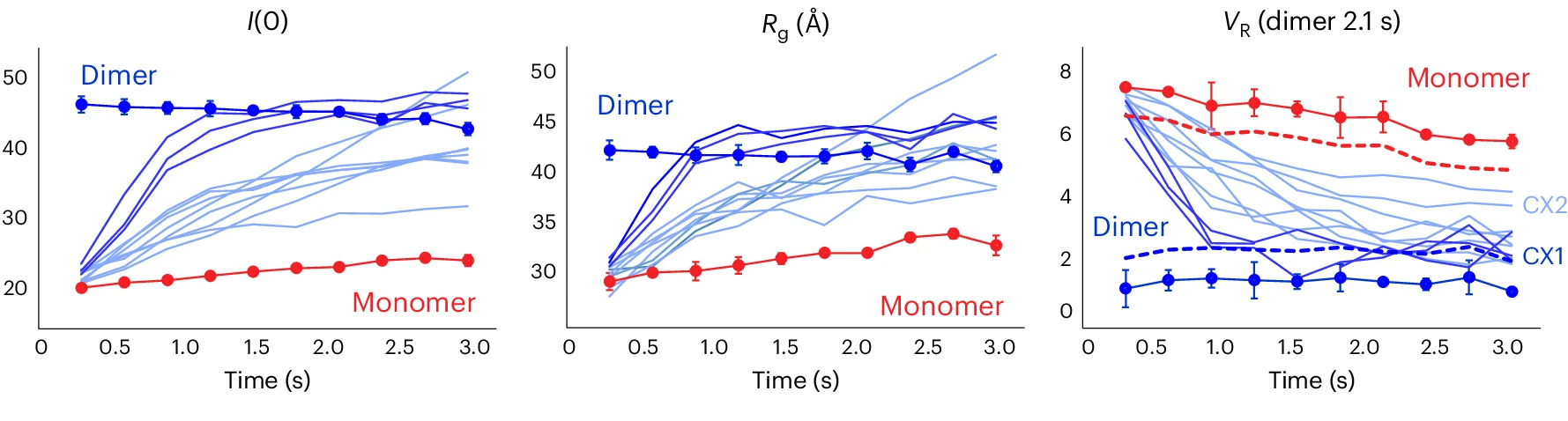
The full conformational landscape
Based on the results, the researchers sorted the small molecules into groups based on how closely each complex resembled the reference dimer structure. The highest-ranking group induced robust AIF dimerization, revealing—via a number of parameters—a rapid transition from monomer to dimer.
As a final step, the researchers crystallized the top small-molecule contenders and analyzed their atomic-level structures using protein crystallography at the National Synchrotron Light Source II and at ALS Beamline 8.3.1. The structures provided further insight into how AIF operates and validated SAXS as a way to uncover structure–activity relationships.
Synchrotrons have a well-recognized role in drug development with crystallography. This work identifies a new potential role with x-ray scattering. The ALS upgrade (ALS-U) could make data collection possible on smaller sample sizes—a challenge where protein quantity is often limiting.
Importantly, this new workflow opens the way to early screening of the full conformational landscape and associated kinetic transitions of a target, expanding the range of accessible clinical targets and ligand binding sites.
Contact: Chris Brosey
Researchers: C.A. Brosey, T.M. Link, R. Shen, D. Moiani, and J.A. Tainer (University of Texas MD Anderson Cancer Center); K. Burnett (Berkeley Lab); G.L. Hura (Berkeley Lab and University of California, Santa Cruz); and D.E. Jones (University of Arkansas for Medical Sciences).
Funding: National Institutes of Health; Cancer Prevention and Research Institute of Texas; Welch Foundation; National Cancer Institute; US Department of Energy (DOE), Office of Science, Basic Energy Sciences (BES) program; DOE Office of Science, Biological and Environmental Research program, Integrated Diffraction Analysis Technologies grant; and Plexxicon, Inc. Operation of the ALS and NSLS-II is supported by DOE BES.
Publication: C.A. Brosey, T.M. Link, R. Shen, D. Moiani, K. Burnett, G.L. Hura, D.E. Jones, and J.A. Tainer, “Chemical screening by time-resolved X-ray scattering to discover allosteric probes,” Nat. Chem. Biol. (2024), doi:10.1038/s41589-024-01609-1.
ALS SCIENCE HIGHLIGHT #506