SCIENTIFIC ACHIEVEMENT
By locking down certain movable parts of a modular drug-building protein, researchers learned new details about how carrier proteins transfer the product protein between modules.
SIGNIFICANCE AND IMPACT
The results, which relied in part on protein crystallography at the Advanced Light Source (ALS), offer insights that could enable scientists to design and create new and improved medicines, such as antibiotics, using synthetic biology.
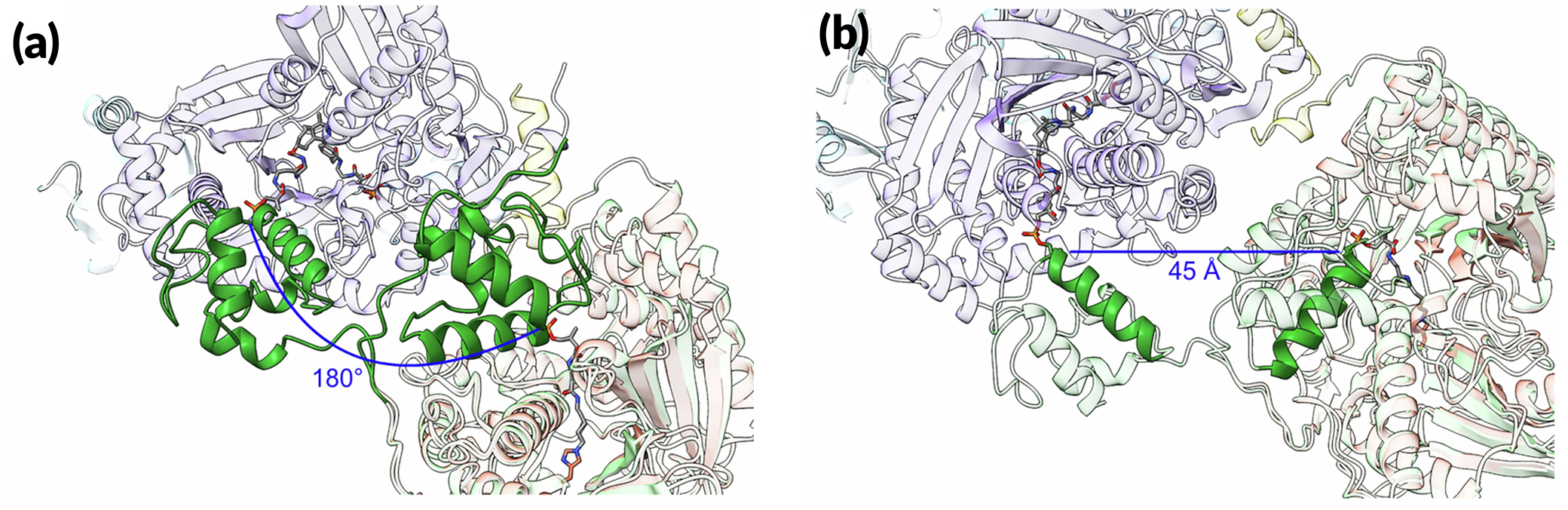
A modular molecular machine
Many drugs used today are peptides (short amino-acid chains) produced on enzymatic assembly lines—i.e., giant proteins known as nonribosomal peptide synthetases (NRPSs), which occur naturally in bacteria. Each “module” on the assembly line installs a single amino-acid building block onto a growing peptide chain. This modularity initially led researchers to believe that NRPS functionality could be mixed and matched to create whatever peptide was desired. However, it turns out that the modules typically cannot be recombined. While each module is essentially self-contained, there are subtle interactions that determine whether modules can work together.

In this work, researchers collected multiple structures—snapshots of the assembly line in action—using both cryogenic electron microscopy (cryo-EM) and protein crystallography, the latter at the ALS. By comparing multiple snapshots, the researchers learned new details about how components of the assembly line work together at the molecular level, a prerequisite to rationally altering the assembly process to speed it up or to make an improved or more potent medicine.
The pieces click into place
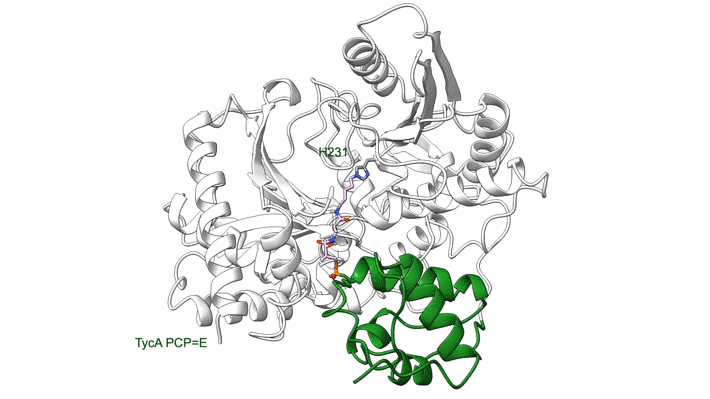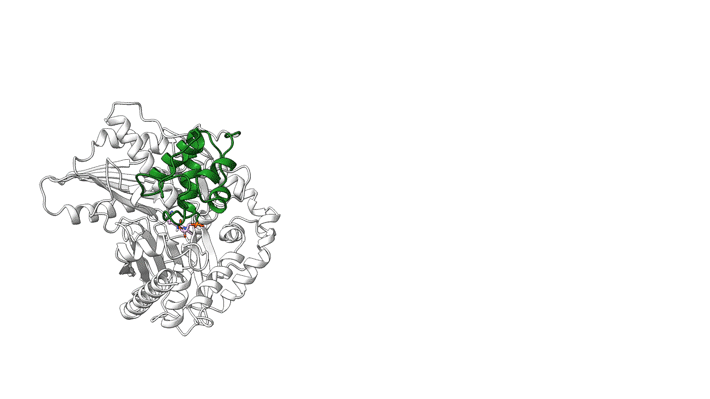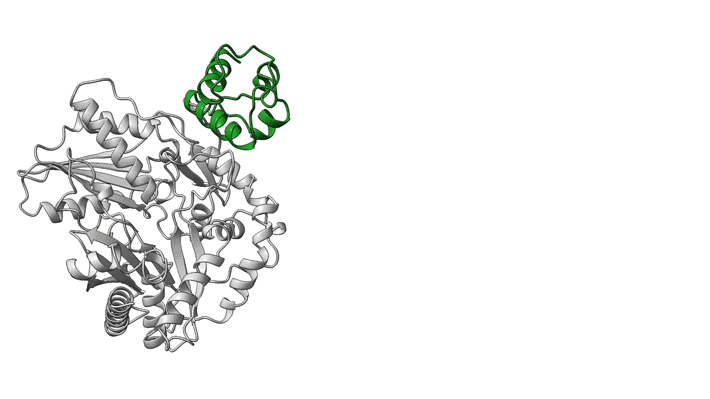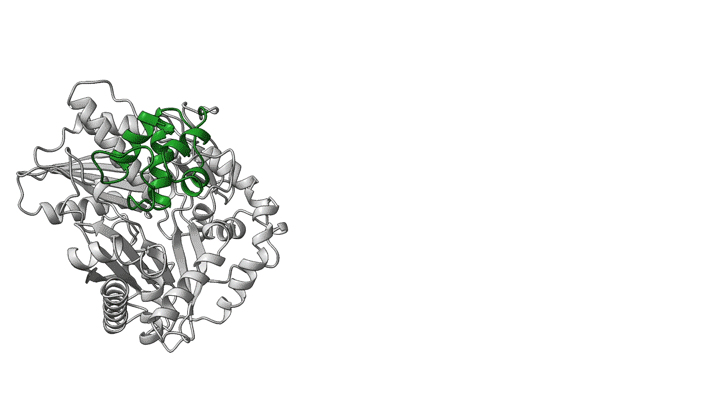
NRPS molecular machines are central to the biosynthesis of important therapeutics such as penicillin, cyclosporine, and daptomycin (to name a few), and there is great interest in designing them to prepare next-generation drugs. Unfortunately, structural information on NRPSs is limited because of significant technical challenges, including highly flexible structural features. For example, within an NRPS module, amino acids are shuttled between functional domains by peptidyl carrier proteins (PCPs), which are analogous to robotic arms that swing around to transfer widgets between the stations of a mechanical assembly line. While tethered to PCPs, amino acids may be passed to and from functional domains, including epimerization (E) domains (which flip chirality if needed) and condensation (C) domains (which link amino acids together).
To better understand elusive NRPS intermodular dynamics, researchers obtained detailed structural data by creating crosslinks that trapped flexible PCPs in various positions. Using a “click chemistry” approach, they attached LEGO-like handles to target molecules, allowing them to “click” together (i.e., form covalent bonds) quickly and easily.
Filling in the gaps
In a model NRPS (tyrocidine A synthetase, produced by the bacterium Brevibacillus parabrevis), the researchers trapped two PCPs within the active site of a C domain and report the high-resolution cryo-EM structure of this complex. Also, at ALS Beamline 5.0.1, protein crystallography was used to obtain the structure of a PCP crosslinked to its E domain.
The combined results reveal that there is enough room in the gap between the first two proteins of the tyrocidine assembly line that the relevant PCP can easily move between the C and E domains without requiring the complex to dissociate. This is important because the function of the E domain—flipping the chirality of the PCP-bound amino acid—is reversible. If the wrong isomer is presented in the C domain, the PCP needs to return to the E domain.
Overall, the structures highlight interfacial mechanics between key modular components and the movement of a carrier protein from one catalytic step to the next. The researchers hope this type of information will help with reengineering NRPSs to unlock the massive diversity of medicinal peptides possible with these pathways.
Contact: Michael Burkart
Researchers: G.W. Heberlig, J.J. La Clair, and M.D. Burkart (University of California San Diego).
Funding: National Institutes of Health. Operation of the ALS is supported by the US Department of Energy, Office of Science, Basic Energy Sciences program.
Publication: G.W. Heberlig, J.J. La Clair, M.D. Burkart, “Crosslinking intermodular condensation in non-ribosomal peptide biosynthesis,” Nature 638, 261 (2025), doi:10.1038/s41586-024-08306-y.
ALS SCIENCE HIGHLIGHT #522