Scientists have gotten their first detailed look at the molecular structure of an enzyme that Nature has been using for eons to help silence unwanted genetic messages. A team of researchers with Berkeley Lab and the University of California, Berkeley, used x-ray crystallography at ALS Beamlines 8.2.1 and 8.2.2 to determine the crystal structure of Dicer, an enzyme that plays a critical role in a process known as RNA interference. The Dicer enzyme is able to snip a double-stranded form of RNA into segments that can attach themselves to genes and block their activity. With this crystal structure, the researchers learned that Dicer serves as a molecular ruler, with a clamp at one end and a cleaver at the other end a set distance away, that produces RNA fragments of an ideal size for gene-silencing.
RNA—ribonucleic acid—has long been known as a multipurpose biological workhorse, responsible for carrying DNA’s genetic messages out from the nucleus of a living cell and using those messages to make specific proteins in a cell’s cytoplasm. In 1998, however, scientists discovered that RNA can also block the synthesis of proteins from some of those genetic messages. This gene-silencing process is called RNA interference and it starts when a double-stranded segment of RNA (dsRNA) encounters the enzyme Dicer.
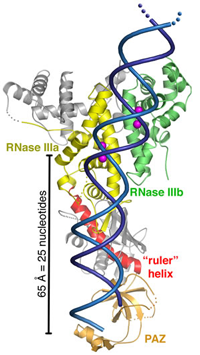
Front view of a ribbon representation of Dicer. The enzyme resembles an axe with the RNA clamp at the handle (the PAZ domain) and the cleaver at the blade (RNase IIIa and IIIb). A flat connector area measuring 65 Å is the ruler portion that is used to measure out segments of 25 nucleotides (bases) in length. A segment of double-stranded RNA (blue) is shown passing through the Dicer enzyme.
Dicer cleaves dsRNA into smaller fragments called short interfering RNAs (siRNAs) and microRNAs (miRNAs). Dicer then helps load these fragments into a large multiprotein complex called RISC, for RNA-Induced Silencing Complex. RISC can seek out and capture messenger RNA (mRNA) molecules (the RNA that encodes the message of a gene) with a base sequence complementary to that of its siRNA or miRNA. This serves to either destroy the genetic message carried by the mRNA outright or else block the subsequent synthesis of a protein.
Until now, it has not been known how Dicer is able to recognize dsRNA and cleave those molecules into products with lengths that are exactly what is needed to silence specific genes. The Berkeley researchers were able to purify and crystallize a Dicer enzyme from Giardia intestinalis, a one-celled microscopic parasite that can infect the intestines of humans and animals. This Dicer enzyme in Giardia is identical to the core of a Dicer enzyme in higher eukaryotes, including humans, that cleaves dsRNA into lengths of about 25 bases.
In this work, the researchers describe a front view of the structure as looking like an axe. On the handle end there is a domain that is known to bind to small RNA products, and on the blade end there is a domain that is able to cleave RNA. Between the clamp and the cleaver is a flat-surfaced region that carries a positive electrical charge. The researchers propose that this flat region binds to the negatively charged dsRNA like biological Velcro, enabling Dicer to measure out and snip specified lengths of siRNA. When you put the clamp, the flat area, and the cleaver together, you get a pretty good idea as to how Dicer works. The research team is now using this structural model to design experiments that might reveal what triggers Dicer into action.
In addition, one size does not fit all for Dicer: different forms of the Dicer enzyme are known to produce different lengths of siRNA, ranging from 21 to 30 base pairs in length or longer. Having identified the flat-surfaced positively charged region in Dicer as the “ruler” portion of the enzyme, the researchers speculate that it may be possible to alter the length of a long connector helix within this domain to change the lengths of the resulting siRNA products. The researchers would like to see what happens when you take a natural Dicer and change the length of its helix.
Contact: Jennifer Doudna
Research conducted by I.J. MacRae and K. Zhou (University of California, Berkeley, and Howard Hughes Medical Institute); F. Li, A. Repic, A.N. Brooks, and W.Z. Cande (University of California, Berkeley); P.D. Adams (Berkeley Lab); and J.A. Doudna (University of California, Berkeley, Howard Hughes Medical Institute, and Berkeley Lab).
Research funding: National Institutes of Health. Operation of the ALS is supported by the U.S. Department of Energy, Office of Basic Energy Sciences.
Publication about this research: I.J. MacRae, K. Zhou, F. Li, A. Repic, A.N. Brooks, W.Z. Cande, P.D. Adams, and J.A. Doudna, “Structural basis for double-stranded RNA processing by dicer,” Science 311, 195 (2006), doi:10.1126/science.1121638.
ALS SCIENCE HIGHLIGHT #120