Sickle cell disease (SCD) affects millions of people worldwide and is one of the most common genetic disorders in the United States. The most-used therapy for the disease is a cytotoxic drug with additional negative side effects and variable patient response. Bay Area biopharmaceutical company Global Blood Therapeutics (GBT) is on a mission to develop a better treatment and is using the ALS to help.
SCD is caused by a single cellular mutation in hemoglobin, a protein in red blood cells that carries oxygen throughout the body. This “sickled” hemoglobin can form stiff rods that adhere to blood vessel walls, causing a blockage that slows or stops the flow of blood. This prevents oxygen from reaching nearby tissues, causing attacks of sudden, severe pain and organ damage. SCD patients also often have trouble producing enough new red blood cells to replace the old cells, resulting in chronic anemia.
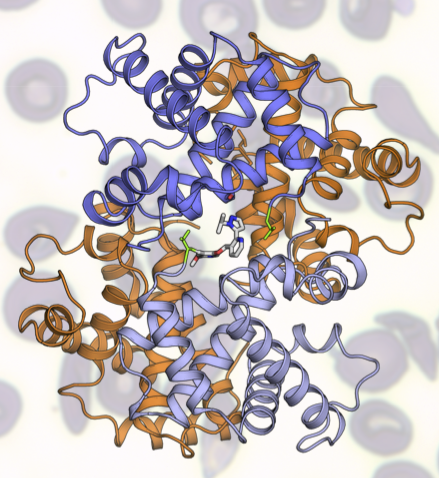
GBT is working to address SCD by developing a therapy that prevents the polymerization of hemoglobin, the key event in promoting the sickle shape. The ALS’s protein crystallography capabilities at Beamline 8.3.1 are critical to their work, giving them insights into potential molecular targets that they wouldn’t be able to access otherwise. Based on results from the ALS, GBT has developed a clear understanding of the molecular mechanism of their compound, GBT440, which works by binding to hemoglobin and increasing the affinity for oxygen, inhibiting hemoglobin polymerization and preventing “sickling.”
On a weekly basis, GBT’s chemistry group develops and sends to the research team different compounds that they’d like the researchers to crystallize and analyze at the ALS. James Partridge, a GBT scientist who has been leading research efforts at the ALS, brings his team to use beam time at the ALS once or twice a month. They then distribute the processed data they’ve collected throughout their research group. Traveling to the ALS is not difficult, as GBT is based in South San Francisco. Partridge is also quite familiar with the ALS, as he was a regular user as a postdoc in David Agard’s lab at UCSF— experience that helped the GBT team get up to speed with ALS tools and technologies quickly.
“Everyone really likes to see the data that emerges because it helps our chemists in designing the compounds; there are always things that can’t be predicted,” says Partridge. “Understanding how the biology works, how the molecule binds, is extremely important to our drug design.”
The GBT team appreciates the fact that at Beamline 8.3.1 they can do their data collection manually rather than it being automated, as it gives them the ability to adapt their research based on results immediately. “It allows us to work very efficiently,” says Partridge. “Beamline staff James Holton, George Meigs, and Jane Tanamachi were so helpful, and they’ve done a great job of maintaining and upgrading the beamline over the years.”
“The trend in crystallography has been towards automation, and there’s plenty of capacity for that so I’ve tried to keep 8.3.1 as a place for interactive science,” says Holton, the Beamline 8.3.1 scientist who worked most closely with GBT. “There’s no substitute for being there with your samples and seeing the data right away and then using that to design your next data set; James [Partridge] and his team make a lot of decisions on the fly as they see their results.”
Beamline 8.3.1 recently upgraded their detector, which has greatly increased the amount of data GBT has been able to collect, says Partridge. “Purchasing the highest technological level of equipment as we can allows us to provide a lot of benefit to industry users,” says Holton. “With our new detector we’ve collected more data in the last 12 months than we had in the previous 16 years. There’s no way GBT would be able to solve so many structures in their own lab.”
Much of the research GBT conducts is confidential, but the company has already published two papers based on their work at the ALS. Holton notes GBT’s generosity in sharing their insights, which is not always common in pharmaceutical research. “They’re a relatively new company with a surprisingly magnanimous mission: trying to solve tough global health problems,” says Holton.
GBT is evaluating the impact of GBT440 in a clinical trial targeted at the treatment of SCD patients across all ages. The clinical trial is currently in phase 3 and has been fast-tracked by the FDA.
Research conducted by: A. Betz, K. Dufu, S.L. Gwaltney II, J. Harris, A. Hutchaleelaha, C. Johnson, Z. Li, B. Metcalf, D. Oksenberg, J. Partridge, M. Patel, P. Rademacher, H. Sham, A. Silva-Garcia, Q. Xu, and C. Yee (Global Blood Therapeutics); Y. Hu, B. Liu, X. Ma, Y. Shan, H. Shi, Q. Xu, L. Zhang, and Y. Zhang (Pharmaron Xi’an Co.); C. Chuang, J. James, Q. Lu, B.P. Morgan, and D. Xu (Cytokinetics, Inc.); S.C. Almo, L. Patskovska, and Y. Patskovsky (Albert Einstein College of Medicine); L. Hua and M.P. Jacobson (UCSF); and K. Paulvannan (Tandem Sciences, Inc.).
Publications about this research: B. Metcalf, C. Chuang, K. Dufu, M.P. Patel, A. Silva-Garcia, C. Johnson, Q. Lu, J.R. Partridge, L. Patskovska, Y. Patrkovsky, S.C. Almo, M.P. Jacobson, L. Hua, Q. Xu, S.L. Gwaltney, C. Yee, J. Harris, B.P. Morgan, J. James, D. Xu, A. Hutchaleelaha, K. Paulvannan, D. Oksenberg, and Z. Li, “Discovery of GBT440, an Orally Bioavailable R-State Stabilizer of Sickle Cell Hemoglobin,” ACS Med. Chem. Lett. 8, 321 (2017). doi: 10.1021/acsmedchemlett.6b00491
Li, J. Partridge, A. Silva-Garcia, P. Rademacher, A. Betz, Q. Xu, H. Sham, Y. Hu, Y. Shan, B. Liu, Y. Zhang, H. Shi, Q. Xu, X. Ma, and L. Zhang, “Structure-Guided Design of Novel, Potent, and Selective Macrocyclic Plasma Kallikrein Inhibitors,” ACS Med. Chem. Lett. 8, 185 (2017). doi: 10.1021/acsmedchemlett.6b00384
Operation of the ALS is supported by the U.S. Department of Science, Office of Science, Basic Energy Sciences Program.