SCIENTIFIC ACHIEVEMENT
Berkeley Lab and Genentech scientists related the internal structures of lipid nanoparticles to their efficacy at drug delivery, using a combination of methods including x-ray scattering at the Advanced Light Source (ALS).
SIGNIFICANCE AND IMPACT
The work promises to expedite the development of drug delivery systems for the treatment of diseases such as COVID-19 and cancer.

Protective packaging for pharmaceuticals
Many diseases can be successfully treated in the simple environment of a cell culture dish, but to successfully treat real people, the drug agent has to take a journey through the infinitely more complex environment within our bodies and arrive, intact, inside the affected cells. This process, called drug delivery, is one of the most significant barriers in medicine.
Researchers from Berkeley Lab and Genentech, a member of the Roche Group, are working together to design more effective lipid nanoparticles (LNPs)—tiny spherical pouches made of fatty molecules (lipids) that encapsulate therapeutic agents until they dock with cell membranes and release their contents. The first drug to use LNPs was approved in 2018, but the delivery method rose to global prominence with the Pfizer and Moderna mRNA COVID vaccines.
LNPs are now being widely explored as a delivery system for vaccines for other infectious diseases or therapeutic vaccines for cancer. The viability of these new applications will depend on how well the lipid envelopes fuse with target cells, how stable the drug–LNP formulations are in storage, and how stable they are in the body. All these properties are controlled by the mixture of molecules used to create the LNP, and the resulting 3D structure of the particle.
Lipid nanoparticles on the fast track
LNPs have four components—ionizable lipids, helper phospholipids, cholesterol, and polyethylene glycol lipids (PEG-lipids)—and each component has different forms. They can be combined in different ratios, leading to an exponential number of possible formulas.

The Genentech researchers addressed these challenges by developing a robot-driven, high-throughput workflow that can generate hundreds of LNP formulations in just a few hours. Additionally, the team incorporated an accelerated process to study how different LNP formulations affect gene expression in their target cells. By combining these techniques, the group is able to screen potential LNPs at an unprecedented rate.
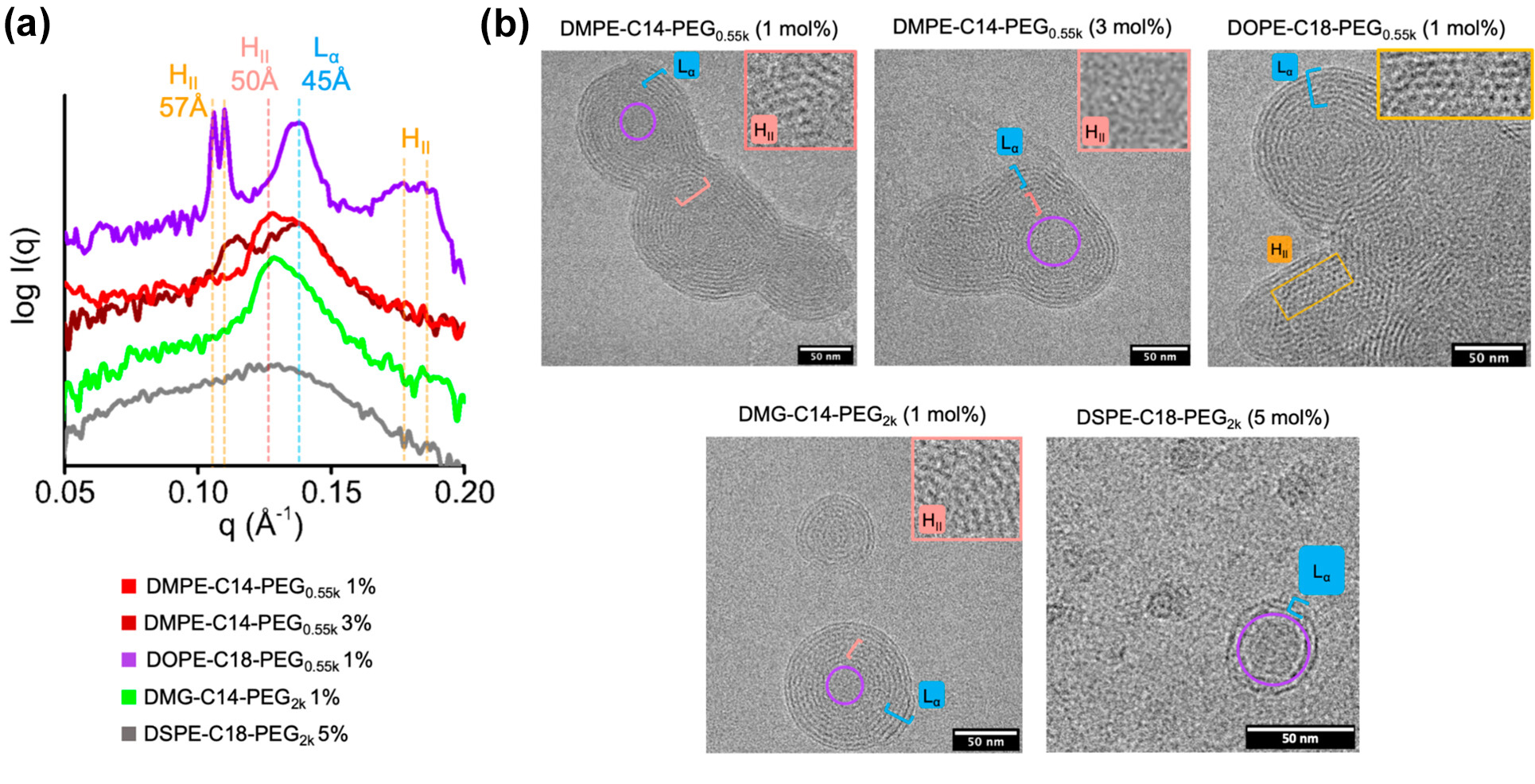
At ALS Beamline 12.3.1 (SIBYLS), Berkeley Lab researchers developed a high-throughput small-angle x-ray scattering (SAXS) platform that allowed the comprehensive mapping of the LNP formulation space, which is currently infeasible using other techniques. In addition, they developed the necessary analysis tools to understand the resulting complex signals. By associating LNP SAXS features with structures observed using cryo-electron microscopy (cryo-EM), the researchers identified critical LNP structural parameters that can be analyzed directly from SAXS data.
Linking structure to activity
The results demonstrated how LNP structure correlates with the activity of its contents, which for this investigation was an anti-sense oligonucleotide (ASO). Oligonucleotides are small snippets of RNA or DNA that block gene expression and are a great way to treat diseases caused by faulty proteins or the over-abundance of a protein. The scientists discovered that ASO-carrying LNPs with neatly ordered, closely packed internal structures led to better silencing of the faulty gene compared with LNPs with a more disordered structure.
The researchers plan to continue using the SAXS beamline to study small details, like how a 1% change in ingredient concentration or using a new machine during production can affect LNP cellular activity, as well as big questions, such as whether LNPs behave differently if they are carrying other cargo types and how they interact with different target cells.
Contacts: Chun-Wan Yen and Greg Hura
Researchers: M. Hammel (Berkeley Lab); Y. Fan, A. Sarode, A.E. Byrnes, N. Zang, P. Kou, K. Nagapudi, D. Leung, C.C. Hoogenraad, T. Chen, and C.-W. Yen (Genentech Inc.); and G.L. Hura (Berkeley Lab and Univ. of California, Santa Cruz).
Funding: Genentech Inc.; National Institutes of Health; and US Department of Energy (DOE), Office of Science, Biological and Environmental Research program. Operation of the ALS is supported by DOE, Office of Science, Basic Energy Sciences program.
Publication: M. Hammel, Y. Fan, A. Sarode, A.E. Byrnes, N. Zang, P. Kou, K. Nagapudi, D. Leung, C.C. Hoogenraad, T. Chen, C.-W. Yen, and G.L. Hura, “Correlating the Structure and Gene Silencing Activity of Oligonucleotide-Loaded Lipid Nanoparticles Using Small-Angle X-ray Scattering,” ACS Nano 17, 11454 (2023), doi:10.1021/acsnano.3c01186.
Adapted from the Berkeley Lab news release, “Breaking Barriers in Drug Delivery with Better Lipid Nanoparticles.”
ALS SCIENCE HIGHLIGHT #491