SCIENTIFIC ACHIEVEMENT
With data obtained at the Advanced Light Source (ALS), researchers gained insight into how an enzyme orchestrates the synthesis of a pectin polymer that imparts strength and flexibility to plant cell walls.
SIGNIFICANCE AND IMPACT
The work could lead to improved biofuel production and guide the design of polymers with tailored functionalities for industrial or biomedical applications.
A peek at pectin production
Pectin is a well-known ingredient that gets jams and jellies to gel. Less well known is the fact that this substance—made up of several types of polysaccharides (sugar-molecule chains)—also acts as a binder between plant cells. By cross-linking with other cell-wall components (such as cellulose and lignin) it imparts strength and flexibility to stems, tree trunks, and branches. In particular, tension wood, which forms where tree trunks or branches bend, is rich in a specialized type of pectin polysaccharide characterized by side chains of galactan, a polymer composed of galactose sugar molecules.
Polysaccharide polymers such as these have enormous potential for reengineering into eco-friendly functional materials, if we can learn how the enzymes that synthesize them work. Toward this end, researchers used x-ray crystallography and solution x-ray scattering at the ALS to determine the structure of galactan synthase 1 (GalS1), an enzyme that catalyzes the growth of galactan side chains. Combined with evolutionary analysis, biochemical studies, and molecular simulations, the results suggest a new model for galactan side-chain synthesis. This knowledge could lead to the development of enzymes designed to process biomass for biofuels or that generate new saccharide structures for industrial or biomedical applications.
ALS protein-structure studies
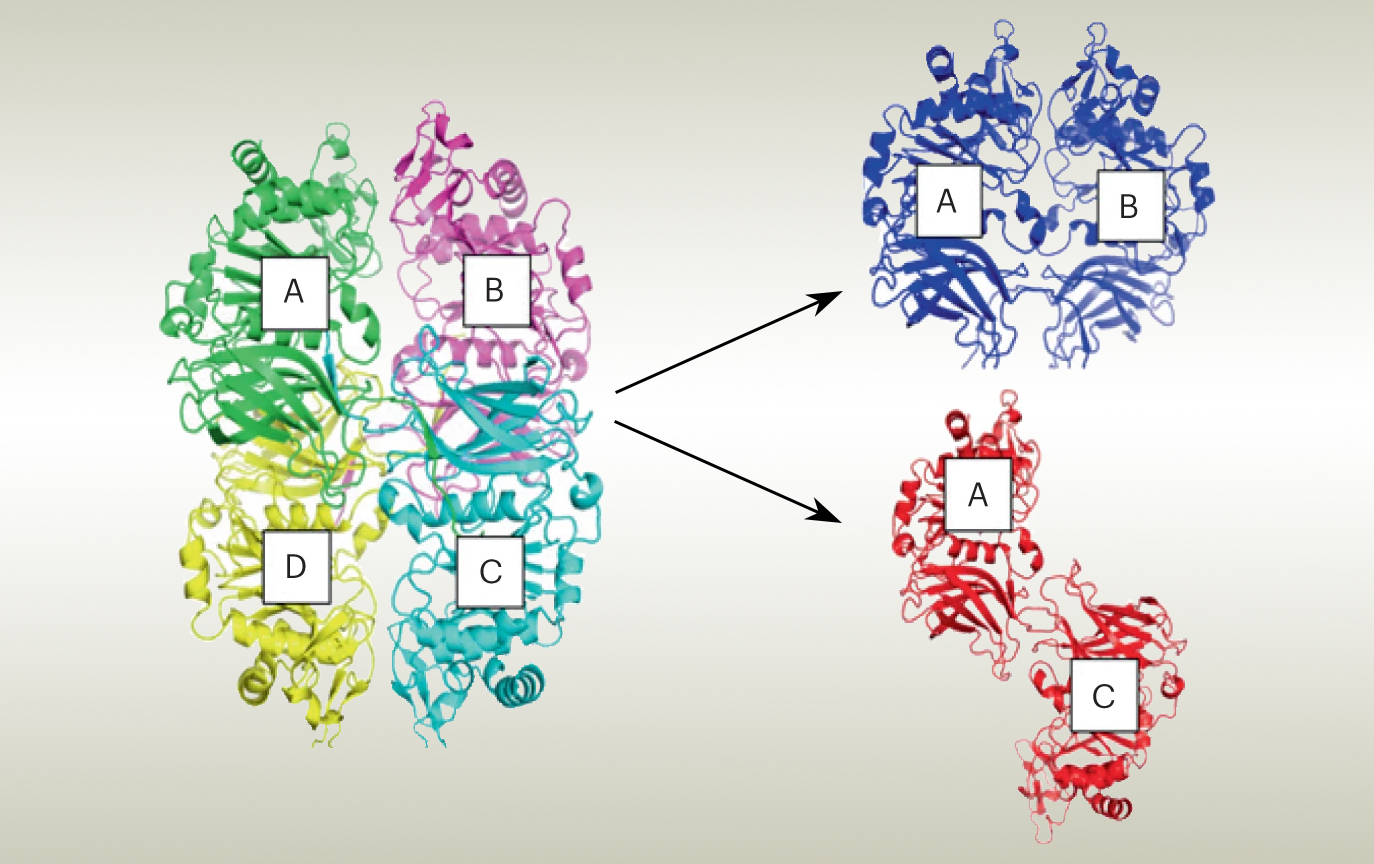
In this work, the researchers used protein crystallography at ALS Beamline 8.2.2 to solve the structure of the GalS1 enzyme. The results revealed a tetrameric structure, with connections between the four proteins. Earlier experiments using size-exclusion chromatography (SEC) with multi-angle light scattering (MALS) indicated that, in solution, the tetrameric complex consists of two dimers in two possible configurations: parallel or antiparallel.
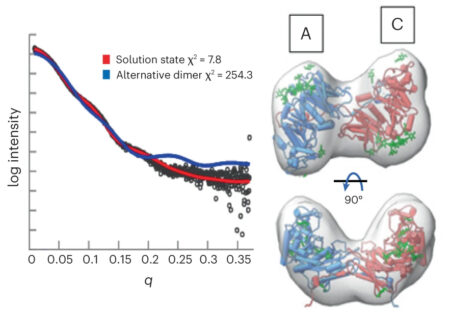
To ascertain the correct orientation, the researchers looked at GalS1 protein complexes in solution at ALS Beamline 12.3.1 (SIBYLS), using small-angle x-ray scattering (SAXS) coupled with SEC-MALS. The experiments yielded information about the shape of the protein’s envelope, which established that the complex forms a dimer in an antiparallel orientation.
Connecting structure to function
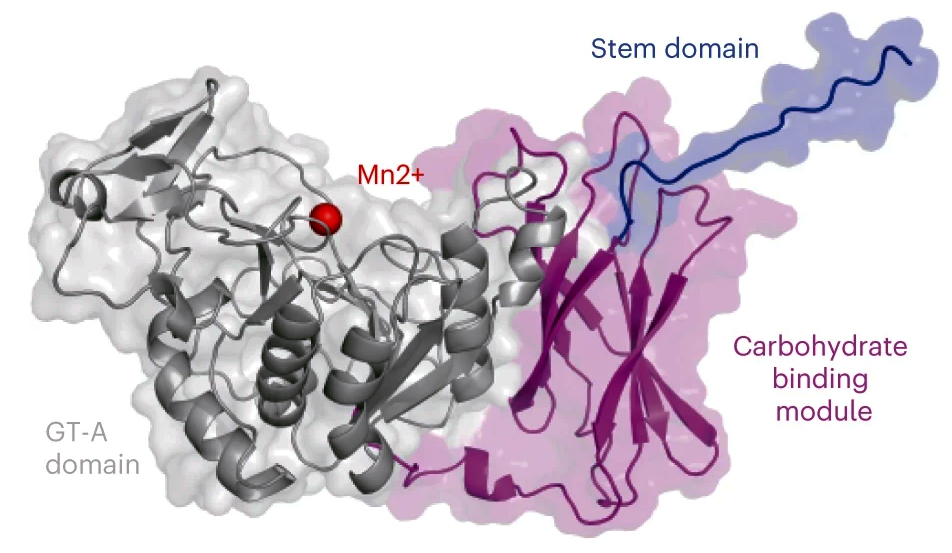
Collectively, the results from the various analyses led the researchers to propose a new model for how GalS1 functions. The structural data established that GalS1 is modular, with three key domains. It has an active region (the glycosyl transferase domain) that extends galactan side chains by forming bonds between galactose molecules. There is a stem region that interacts across chains and is essential for maintaining stability and activity. Finally, the researchers identified a unique noncatalytic carbohydrate binding module (CBM) that represents a new family of plant-specific CBMs in carbohydrate-active enzymes. In the researchers’ model, this novel CBM functions to bring the GalS1 enzyme in proximity to the pectin backbone, potentially targeting sparsely occupied regions.
The next step for the researchers would be to study this process in vivo, using different types of synthetic biology genome-engineering approaches to, for example, put mutant versions of the enzyme back into model species to see if it’s possible to manipulate plant structure. In the future, they hope to use structural studies to develop targeted engineering strategies to modify enzymes such as GalS1 to enzymatically generate new saccharide structures.
Contact: Breeanna Urbanowicz
Researchers: P.K. Prabhakar and B.R. Urbanowicz (Univ. of Georgia and Oak Ridge National Laboratory); J.H. Pereira, W. Shao, and M. Hammel (Berkeley Lab); R. Taujale, D. Chapla, J.-Y. Yang, K.W. Moremen, and N. Kannan (Univ. of Georgia); V.S. Bharadwaj and Y.J. Bomble (National Renewable Energy Laboratory); and P.D. Adams and H.V. Scheller (UC Berkeley and Berkeley Lab).
Funding: U.S. Department of Energy (DOE), Office of Science, Biological and Environmental Research program; Howard Hughes Medical Institute; and National Institutes of Health. Operation of the ALS is supported by DOE, Office of Science, Basic Energy Sciences program.
Publication: P.K. Prabhakar, J.H. Pereira, R. Taujale, W. Shao, V.S. Bharadwaj, D. Chapla, J.-Y. Yang, Y.J. Bomble, K.W. Moremen, N. Kannan, M. Hammel, P.D. Adams, H.V. Scheller, and B.R. Urbanowicz, “Structural and biochemical insight into a modular β-1,4-galactan synthase in plants,” Nat. Plants 9, 486 (2023), doi:10.1038/s41477-023-01358-4.
ALS SCIENCE HIGHLIGHT #484