SCIENTIFIC ACHIEVEMENT
Researchers isolated human-derived antibodies that protect against malaria, and protein-structure studies revealed the antibodies’ site of attack.
SIGNIFICANCE AND IMPACT
The discovery paves the way for the development of a more effective and practical human vaccine for malaria, a disease responsible for half a million deaths worldwide each year.
The need for a malaria vaccine
Malaria kills about 445,000 people a year, mostly young children in sub-Saharan Africa, and sickens more than 200 million. It’s caused by a parasite, Plasmodium falciparum (Pf), and is spread to humans through the bite of an infected Anopheles mosquito.
The parasite’s complex life cycle and rapid mutations have long challenged vaccine developers. Only one experimental vaccine, known as RTS,S, has progressed to a Phase 3 clinical trial (testing on large groups of people for efficacy and safety). To elicit an immune response, this vaccine uses a fragment of circumsporozoite protein (CSP), which covers the malaria parasite in its native conformation. However, the trial results showed that RTS,S is only moderately effective, protecting about one-third of the young children who received it over a period of four years.
A new antibody is isolated
In the work described here, researchers isolated a new antibody, CIS43, from the blood of a human volunteer who received an experimental vaccine made from whole, weakened malaria parasites. The use of a whole-parasite vaccine allowed the isolation of antibodies against CSP. The CIS43 antibody in particular proved to be highly effective at preventing malaria infection following transfer into mice prior to exposure.
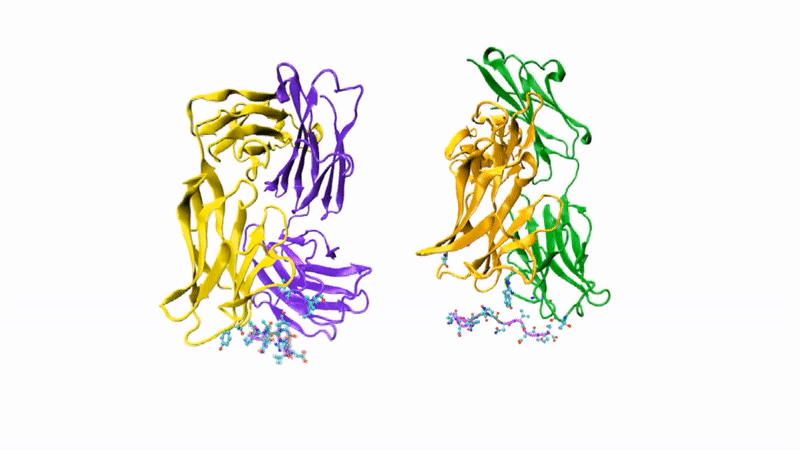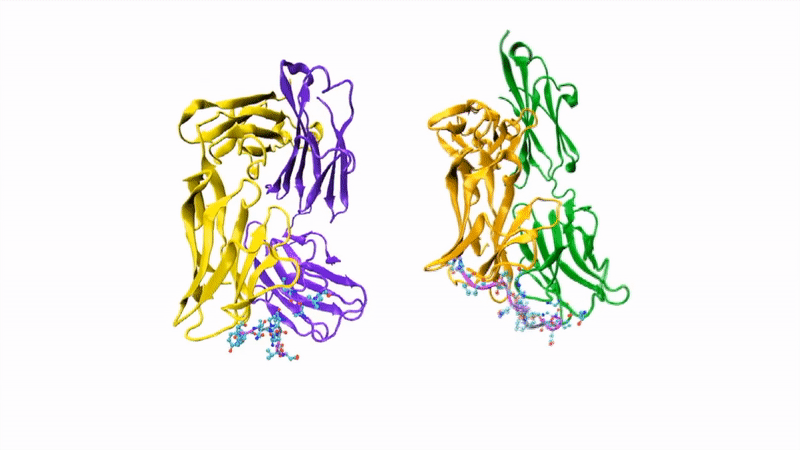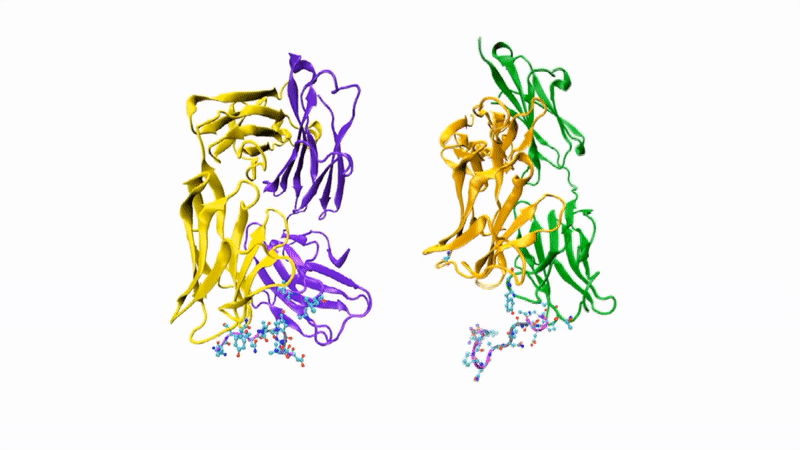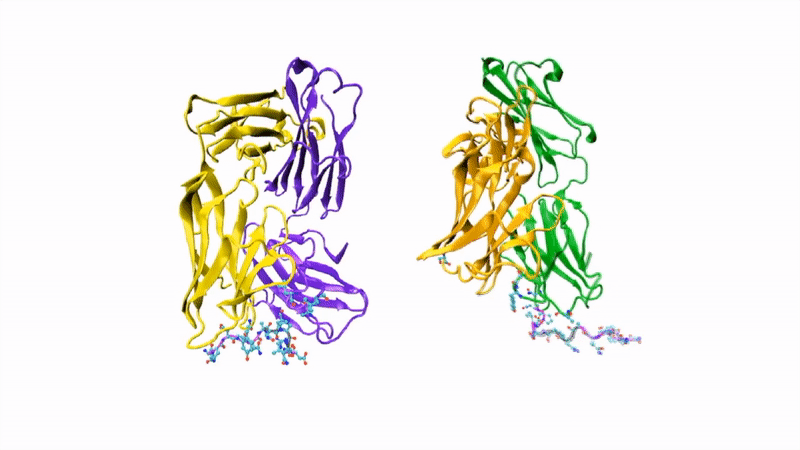
The researchers showed that CIS43 strongly binds to a novel site at the junction of CSP’s N-terminus and repeat region (the “junctional epitope”). The antibody and the junctional epitope were subjected to a number of biophysical and structural analyses, with the ultimate goal of rationally designing a vaccine with improved efficacy and practicality.
In the groove
For structural studies, the researchers co-crystallized CIS43 with various peptides (amino-acid sequences) from CSP, including the junctional epitope. Protein crystallography at ALS Beamlines 5.0.1 and 5.0.2 revealed that the peptides fit into a groove formed between two parts of the antibody (the heavy chain and the light chain), along with details of the interactions. For comparison, the researchers also co-crystallized the peptides with antibody CIS42, which also showed specificity to the junctional epitope but had limited neutralizing function in vivo. Of note, CIS43’s angle of binding to the junctional epitope differed from CIS42’s.
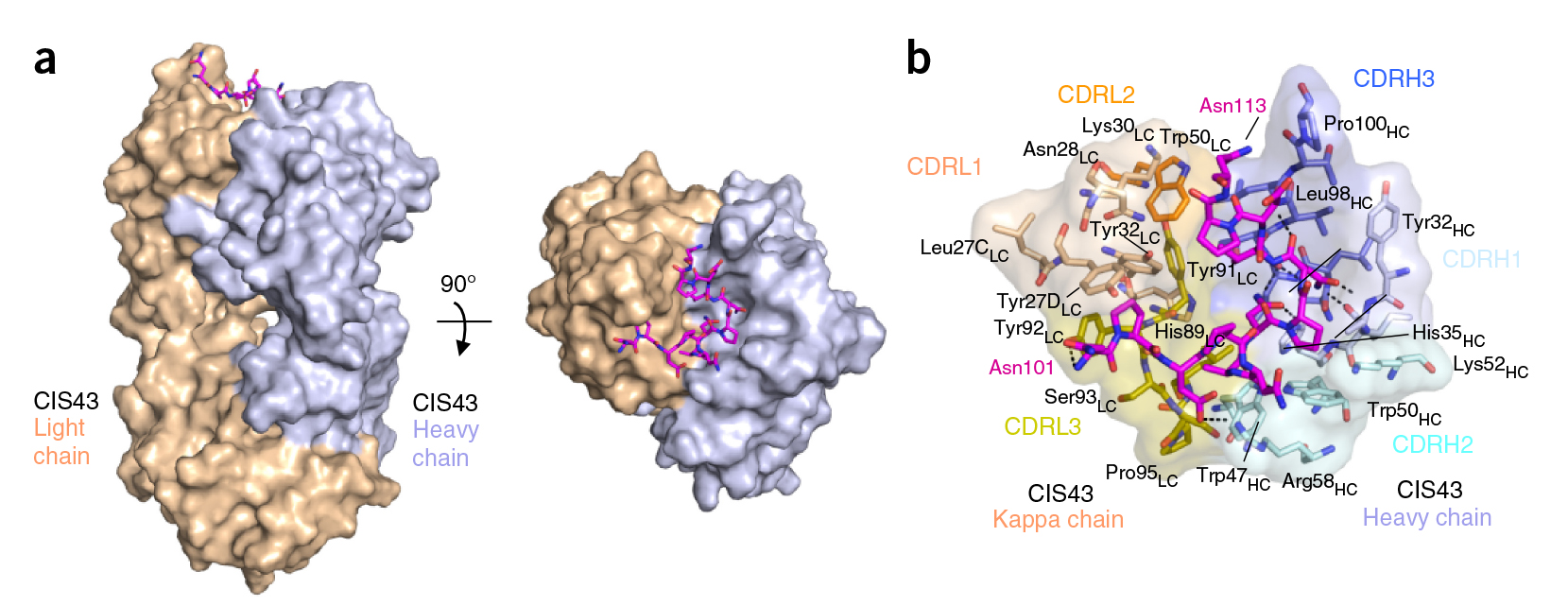
Target acquired
In general, it was found that CIS43 binds strongly to a specific epitope of CSP—peptide 21—that occurs only once along the length of the protein. This CIS43-binding epitope is conserved across 99.8 percent of all known strains of Pf, making CIS43 potentially useful as a preventive antibody against all Pf strains. Moreover, peptide 21 provides a conserved target for next-generation experimental malaria vaccines.
The researchers plan to assess the safety and protective efficacy of CIS43 next year in controlled human malaria infection challenge trials. If the tests go well, CIS43 could be developed as a prophylactic measure to prevent infection for several months after administration. Such a prophylactic antibody could be useful for tourists, health-care workers, military personnel, or others who travel to areas where malaria is common. Moreover, if the antibody prevents malaria infection for up to six months, it might be combined with antimalarial drugs and be deployed as part of mass drug administration efforts that potentially could eliminate the disease in malaria-endemic regions.
Contact: Marie Pancera
Researchers: N.K. Kisalu, A.H. Idris, B.J. Flynn, J.R. Francica, M. Kanekiyo, A.K. Wheatley, A.B. McDermott, S.K. Farney, G.-Y. Chuang, B. Zhang, P.D. Kwong, and R.A. Seder (National Institutes of Health); C. Weidle and M. Pancera (Fred Hutchinson Cancer Research Center); Y. Flores-Garcia, A. Schön, E. Freire, J. Gregory, P. Sinnis, and F. Zavala (Johns Hopkins University); B.K. Sack, S. Murphy, and S.H.I. Kappe (Seattle Biomedical Research Institute); A.B. Miller, S. March, and S.N. Bhatia (Massachusetts Institute of Technology); H.-X. Liao, B.F. Haynes, K. Wiehe, A.M. Trama, K.O. Saunders, M.A. Gladden, A. Monroe, and M. Bonsignori (Duke University); N. Kc, S. Chakravarty, B.K.L. Sim, and S.L. Hoffman (Sanaria Inc.).
Funding: National Institutes of Health, National Science Foundation, and Fred Hutchinson Cancer Research Center. Operation of the ALS is supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences Program (DOE BES).
Publication: N.K. Kisalu, A.H. Idris, C. Weidle, Y. Flores-Garcia, B.J. Flynn, B.K. Sack, S. Murphy, A. Schön, E. Freire, J.R. Francica, A.B. Miller, J. Gregory, S. March, H.-X. Liao, B.F. Haynes, K. Wiehe, A.M. Trama, K.O. Saunders, M.A. Gladden, A. Monroe, M. Bonsignori, M. Kanekiyo, A.K. Wheatley, A.B. McDermott, S.K. Farney, G.-Y. Chuang, B. Zhang, N. Kc, S. Chakravarty, P.D. Kwong, P. Sinnis, S.N. Bhatia, S.H.I. Kappe, B.K.L. Sim, S.L. Hoffman, F. Zavala, M. Pancera, and R.A. Seder, “A human monoclonal antibody prevents malaria infection by targeting a new site of vulnerability on the parasite,” Nat. Med. 24, 408 (2018), doi:10.1038/nm.4512.
Adapted from the Fred Hutchinson Cancer Research Center press release, “New antibody and unique binding site offer possible paths to malaria prevention” by Mary Engel, and the National Institutes of Health news release, “Newly described human antibody prevents malaria in mice.”
ALS SCIENCE HIGHLIGHT #378