Scientists have discovered a primitive form of rubisco, a photosynthesis enzyme that has helped shape life on Earth. Detailed information about its structure, determined using complementary techniques at the ALS, will help scientists understand how carbon-fixing organisms oxygenated the atmosphere and how modern plants evolved. Read more »
Jennifer Doudna and the Nobel Prize: The Advanced Light Source Perspective
The 2020 Nobel Prize in Chemistry was awarded to Jennifer Doudna and Emmanuelle Charpentier for the development of a world-changing gene-editing technology. At the ALS, Doudna’s work on CRISPR-Cas9 was enabled by many visionary people with innovative ideas, implemented in support of a world-class structural biology program. Read more »![]()
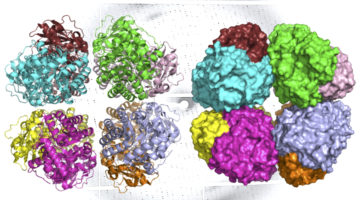
Study Finds ‘Missing Link’ in the Evolutionary History of Carbon-Fixing Protein Rubisco
Scientists discovered an ancient form of rubisco, the most abundant enzyme on Earth and critical to life as we know it. Found in previously unknown environmental microbes, the newly identified rubisco provides insight into the evolution of the photosynthetic organisms that underlie the planet’s food chains. Read more »
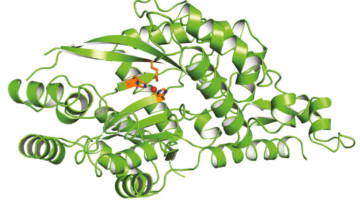
Missing Lysine Link Could Improve Plant-Based Nutrition
To engineer crops with higher levels of the important amino acid, lysine, researchers solved the structure of an enzyme that helps break down lysine in plants. A fuller understanding of the factors affecting lysine levels should aid in the successful development of stable high-lysine crops to combat malnutrition globally. Read more »
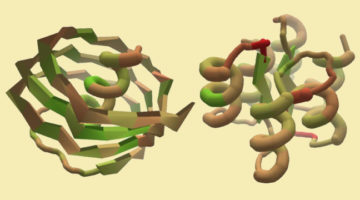
A Citizen-Science Computer Game for Protein Design
Using the computer game, “Foldit,” nonexpert citizen scientists designed new proteins whose structures, verified at the ALS, were equivalent in quality to and more structurally diverse than computer-generated designs. The work shows the potential of using crowd-based creativity in the design of new proteins for fighting illness and disease. Read more »![]()
![]()
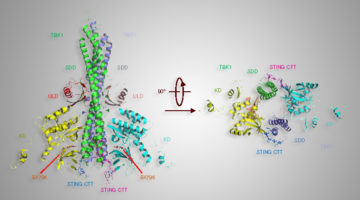
X-Ray Studies Key in Study Relating to Immune System-Signaling Protein
A grouping of amino acids—part of an important signaling protein, STING—plays an important role in activating the immune system. A study conducted through the Collaborative Crystallography program at the ALS confirmed how this part of the STING protein helps to bind a protein-modifying enzyme associated with autoimmune diseases and some cancers. Read more »
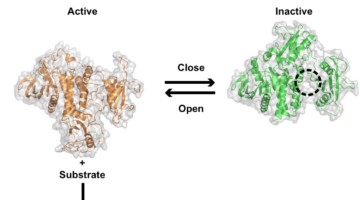
Locking Protein Structure to Close the Door on Cancer
While the SHP2 protein helps regulate cellular activity, mutations in its structure can lead to cancer. X-ray crystallography at the ALS and SSRL has revealed differences between normal and mutated SHP2, as well as how it binds to certain cancer drugs. These structural insights open the door to new types of cancer therapy. Read more »
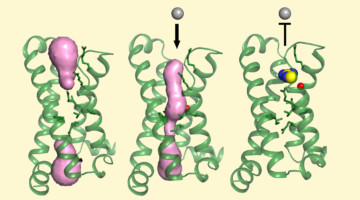
Structure Reveals Mechanism Behind Periodic Paralysis
X-ray crystallography of a membrane protein provided a structural understanding of how a single mutation can result in periodic muscle paralysis. The results suggest possible drug designs that could provide relief to patients with a genetic disorder that causes them to be overcome suddenly with profound muscle weakness. Read more »![]()
![]()
NIH Grant Will Enhance Structural Biology Research Experience for ALS Users
A recently awarded National Institutes of Health (NIH) grant will help integrate existing structural biology resources at the ALS to better serve users. The funds will help establish a centralized collaborative mechanism, called ALS-ENABLE, that will guide users through the most appropriate routes for answering their biological questions. Read more »
ALS Work Highlighted in DOE Top 40 Countdown
To celebrate DOE’s 40th anniversary (October 1, 2017), the Office of Science (SC) collected 40 scientific milestones from the previous 40 years, each one supported by SC. The ALS played a key role in two of the milestones: 2005 (ribosome) and 2009 (topological materials). Read more »