SCIENTIFIC ACHIEVEMENT
X-ray diffraction data, collected at the Advanced Light Source (ALS) and other Department of Energy light sources, revealed the crystal structure of CMX410, a new compound that targets a key enzyme (Pks13) in the cell membrane of the bacterium responsible for tuberculosis (TB).
SIGNIFICANCE AND IMPACT
CMX410 is a promising new candidate to treat TB, including multidrug-resistant strains.
New treatments needed to tackle an old foe
TB is a deadly infectious disease caused by the bacterium Mycobacterium tuberculosis (Mtb). According to the World Health Organization, an estimated 10.8 million people contracted TB globally in 2023, and 1.25 million died from the disease. While antibiotics are effective for drug-sensitive TB cases, multidrug-resistant Mtb strains can evade common drug therapies.
Drug-resistant cases require a regimen that is often more expensive, toxic, and time-intensive. Patients are required to take six or more medications daily for up to 20 months. New approaches are urgently needed to shorten the course of drug interventions and address widespread multidrug-resistant strains.
A multi-institutional study led by researchers at Texas A&M University and the Calibr-Skaggs Institute for Innovative Medicine sought to find new treatments to address multidrug-resistant TB. The team screened a library of 406 compounds that belong to an active class of molecules [i.e., sulfur fluoride exchange (SuFEx)] to evaluate their efficacy against Mtb. The team developed one promising compound into CMX410, which targets Pks13, an enzyme essential for microbial cell wall biosynthesis.
Crystallography revealed the reaction mechanism
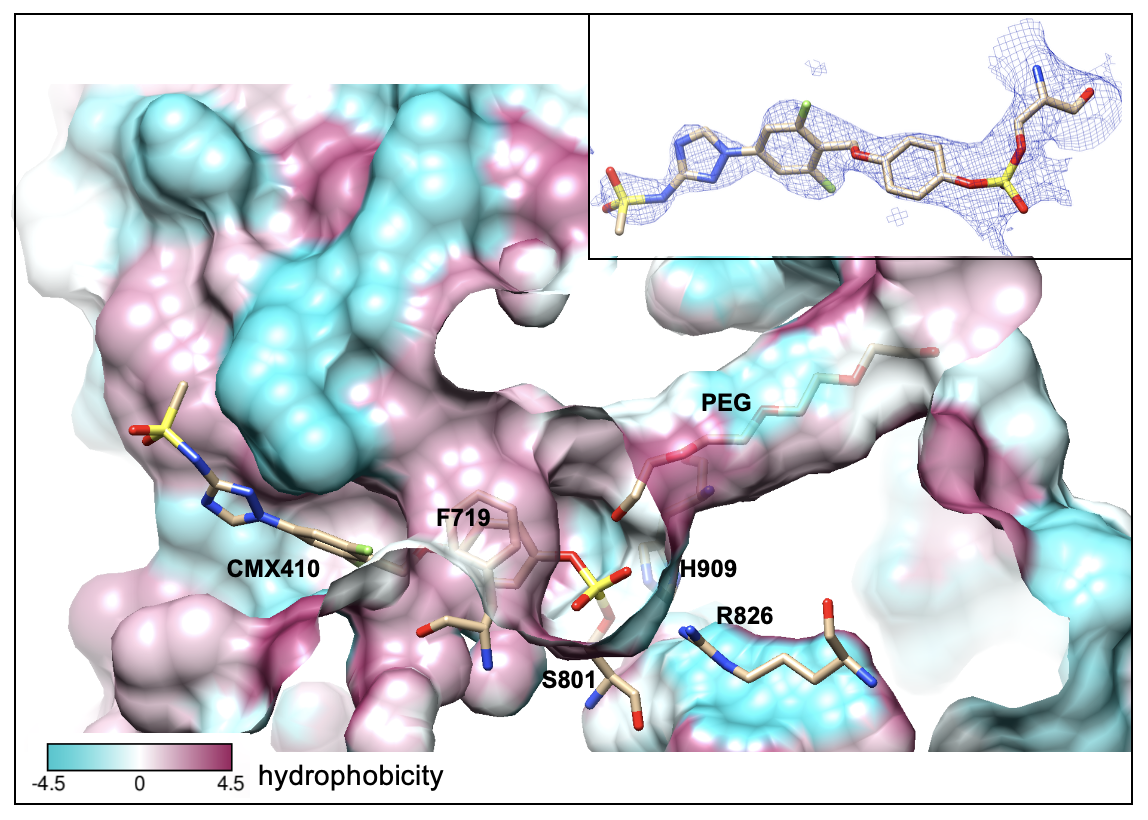
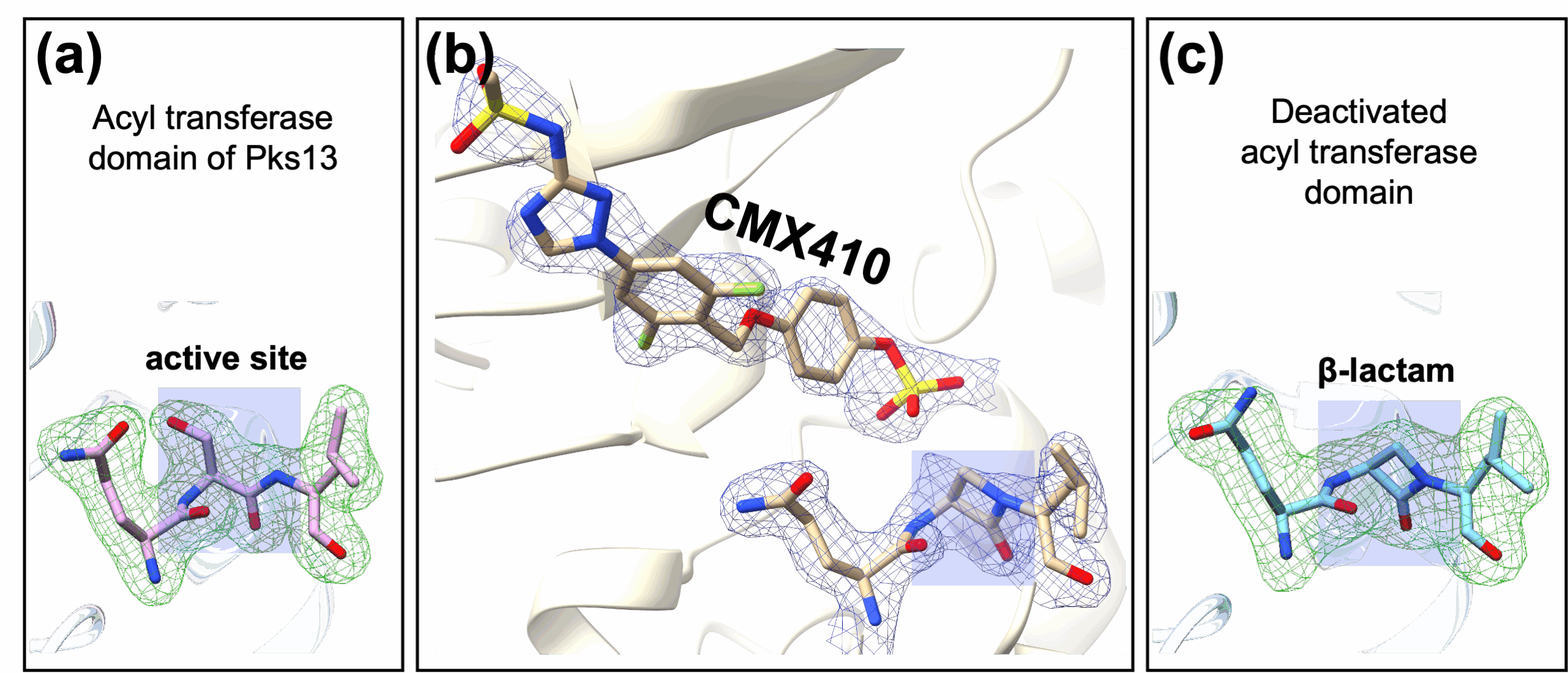
To understand how CMX410 functions, the researchers solved its crystal structure using protein crystallography (PX) at Beamline 5.0.1 and Beamline 5.0.2, which are part of the Berkeley Center for Structural Biology (BCSB) at Lawrence Berkeley National Laboratory (Berkeley Lab). The diffraction data revealed the compound’s interaction with Pks13. The team found that over time, CMX410 reacts with the active site of Pks13, removing an oxygen atom from the enzyme. With the oxygen removed, the backbone of the enzyme forms a β-lactam structure, permanently disabling it.
The researchers aimed to capture the reaction steps to understand the mechanism of inhibition. Their structural work focused on the acyl transferase (AT) domain of Pks13 where the reaction occurs. This required sampling many conditions to “trap” the intermediates as the inhibitor reacted with the enzyme. Thus, the team collected data from many crystals of CMX410 and Pks13 to detail every reaction step. They complemented the ALS diffraction data with data obtained at Argonne National Laboratory’s Advanced Photon Source (APS) and Brookhaven National Laboratory’s National Synchrotron Light Source-II (NSLS-II).
Targeting new pathways to potential treatments
Mtb treatments have targeted the bacterium’s cell wall since the 1960s, but many of these drugs are losing their efficacy due to widespread resistance. CMX410 interferes with a pathway in cell wall metabolism that is not currently targeted by available drugs. CMX410 is active against drug-sensitive and drug-resistant strains of the bacterium. It has also been proven to be effective and safe in multiple animal models of infection.
TB is a complex disease that requires a regimen of multiple drugs. Additional testing is ongoing to determine how CMX410 fits into established TB drug combinations and to complete the safety profile required for drug candidates to progress into clinical practice. The results of this study shed light on the potential of this class of SuFEx-based inhibitors to treat TB and in the broader field of drug discovery.
Contacts: Inna V. Krieger and Daniil Prigozhin
Funding: National Institutes of Health (NIH) TB Structural Genomics grant, TB Drug Accelerator Program of the Gates Foundation, the Welch Foundation, and NIH National Institute of Allergy and Infectious Diseases. Operations of the ALS, APS, and NSLS-II are supported by the Department of Energy, Basic Energy Sciences.
Researchers: I.V. Krieger, S. Tang, D. Selle, T.R. Ioerger, and J.C. Sacchettini (Texas A&M University); P. Sukheja, B. Yang, A. Woods, M.B. Harbut, D. Liu, B. Tsuda, B. Qin, G.A.L. Bare, V. Chi, A. Cascioferro, E. Kundrick, K-J. Lee, S. Li, S.B. Joseph, M.S. Love, H.M. Petrassi, A.K. Chatterjee, and C.W. McNamara (Calibr-Skaggs Institute for Innovative Medicines); C. Engelhart, D. Schnappinger (Weill Cornell Medicine); P. Kumar, P. Singh, D. Alland (Rutgers University); G. Li, K.B. Sharpless (The Scripps Research Institute); J. Gambacurta, J. Hvizdos, M. Reagan, I.L. Jones, W. Reiley (Trudeau Institute); L.M. Massoudi, L.K. Woolhiser, G.T. Robertson (Colorado State University); D. Reddy Kandula, J.W. McCabe (AB Sciex LLC); T. Guo, J. Dong (Shanghai Jiao Tong University); H.I. Boshoff (National Institute of Allergy and Infectious Diseases); K. Mdluli (Gates Medical Research Institute); and P.G. Schultz (Calibr-Skaggs Institute for Innovative Medicines and The Scripps Research Institute)
Publication: I.V. Krieger, P. Sukheja, B. Yang, S. Tang, D. Selle, A. Woods, C. Engelhart, P. Kumar, M.B. Harbut, D. Liu, B. Tsuda, B. Qin, G.A.L. Bare, G. Li, V. Chi, J. Gambacurta, J. Hvizdos, M. Reagan, I.L. Jones, L.M. Massoudi, L.K. Woolhiser, A. Cascioferro, E. Kundrick, P. Singh, W. Reiley, T.R. Ioerger, D. Reddy Kandula, J.W. McCabe, T. Guo, D. Alland, H.I. Boshoff, D. Schnappinger, G.T. Robertson, K. Mdluli, K-J. Lee, J. Dong, S. Li, P.G. Schultz, S.B. Joseph, M.S. Love, K.B. Sharpless, H.M. Petrassi, A.K. Chatterjee, J.C. Sacchettini, and C.W. McNamara. “SuFEx-based antitubercular compound irreversibly inhibits Pks13,” Nature 645, 755–763 (2025), doi: 10.1038/s41586-025-09286-3.
ALS SCIENCE HIGHLIGHT #532