Synthetic radionuclides, such as the transuranic actinides plutonium, americium, and curium, present severe health threats as contaminants, and understanding the scope of the biochemical interactions involved in actinide uptake into cells is instrumental in managing human contamination.
Recently, scientists have reported that an iron-binding protein called siderocalin can also bind and transport actinides into cells, a major advance in understanding the biological chemistry of radioactive metals. The research, which included structural biology studies at the ALS, opens up new avenues of research into strategies for remedial action in the event of possible human exposure to nuclear contaminants.
The research group, from Berkeley Lab and the Fred Hutchinson Cancer Research Center, has already developed a compound to sequester actinides and expel them from the body. They have put it in a pill form that can be taken orally, a necessity in the event of radiation exposure amongst a large population. Last year, the FDA approved a clinical trial to test the safety of the drug, and they are seeking funding for the tests. However, a basic understanding of how actinides act in the body is still needed. Although actinides are known to rapidly circulate and deposit into major organs such as bone, liver, or kidney after contamination, the specific molecular mechanisms associated with mammalian uptake of these toxic heavy elements remain largely unexplored.
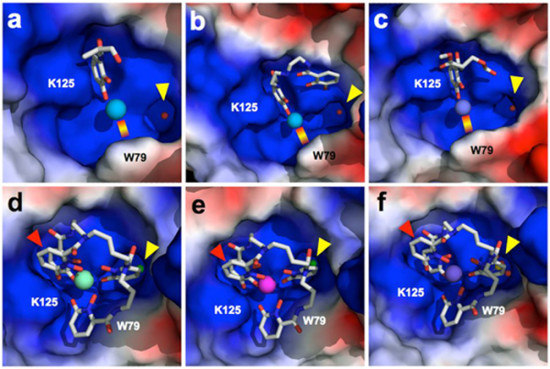
Typically, the mechanisms proposed for mammalian actinide transport have involved proteins that bind their targets directly. Siderocalin, an essential antibacterial protein that sequesters iron, binds indirectly, through tight complexes with a chelator (a molecule that forms multiple bonds with a metal ion). The researchers hypothesized that the metal ion could be substituted without significantly affecting recognition, potentially uncovering cellular trafficking pathways for other elements or enabling novel applications.
At Beamlines 5.0.1 and 5.0.2, the team used crystallography to characterize complexes of siderocalin and transuranic actinides. The results confirmed specific binding, uncovered the details of protein–ligand and chelator–metal coordination, and highlighted differences in the recognition of nontransition metals with natural or synthetic chelators. These are the first protein structures containing thorium or the transuranic elements plutonium, americium, or curium. Until this work, there were no structures in the Protein Data Bank with those elements. The scientists also used cultured kidney cells to demonstrate the role of siderocalin in facilitating the uptake of the metal ions in cells.
By revealing a new pathway for the intracellular delivery of the radioactive toxic metal ions, the results could help scientists to develop new strategies to counteract actinide exposure. For example, instead of binding and expelling radionuclides from the body, one could possibly block the uptake.
The researchers also made the unexpected finding that siderocalin can act as a “synergistic antenna” that sensitizes the luminescence of actinides and lanthanides. They showed that adding the protein enhances the sensitization pathways, making it much brighter. This is a new mechanism that hasn’t yet been explored and could be very useful; it could have applications down the line for diagnostics and bioimaging.
The researchers note that a study like this would have been possible in very few other places. Very few facilities have the capability to combine the different approaches and techniques—the spectroscopy techniques at the ALS, the ability to handle heavy elements that are radioactive, plus the chemical and biological tools available onsite. Beyond elucidating contamination pathways, these results also highlight new potential uses of siderocalin, ranging from the design of photoluminescent materials, to the separation of lanthanides and actinides in the nuclear fuel process, to the functionalization of targeting biologics for imaging and therapeutic radionuclide delivery, by enabling the selective and stoichiometric chelation of a wide range of elements.

Contact: Rebecca Abergel
Research conducted by: B.E. Allred, S.S. Gauny, D.D. An, C.Y. Ralston, M. Sturzbecher-Hoehne, and R.J. Abergel (Berkeley Lab) and P.B. Rupert and R.K. Strong (Fred Hutchinson Cancer Research Center).
Research funding: National Institutes of Health and U.S. Department of Energy (DOE), Office of Basic Energy Sciences (BES). Operation of the ALS is supported by DOE BES.
Publication about this research: B.E. Allred, P.B. Rupert, S.S. Gauny, D.D. An, C.Y. Ralston, M. Sturzbecher-Hoehne, R.K. Strong, and R.J. Abergel, “Siderocalin-mediated recognition, sensitization, and cellular uptake of actinides,” PNAS 112, 10342 (2015).
ALS SCIENCE HIGHLIGHT #323