A pathway to more effective and efficient synthesis of pharmaceuticals and other flow-reactor chemical products has been opened by a study in which, for the first time, the catalytic reactivity inside a microreactor was mapped in high resolution from start to finish. The formation of different chemical products during the reactions was analyzed in situ using infrared microspectroscopy, while the state of the catalyst along the flow reactor was determined using x-ray absorption spectroscopy. The results not only provide a better understanding of the chemistry behind the catalytic reactions, they also reveal opportunities for optimization, resulting in better catalytic performances.
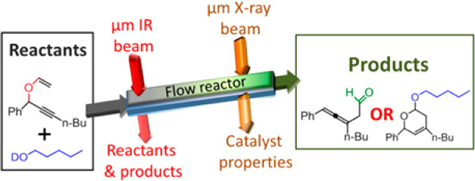
Catalysts—substances that speed up the rates of chemical reactions without themselves being chemically changed—are used to initiate virtually every manufacturing process that involves chemistry. For this study, the researchers used a catalyst of gold nanoclusters to produce dihydropyran, an organic compound whose formation involves multiple reactant steps. Each of the reactants and products shows a distinguishable infrared signature, allowing their evolution into the final product to be precisely monitored with infrared spectroscopy.
Although other (non-synchrotron) spectroscopic tools have the ability to tune into such chemical signatures, they usually do not have sufficient spatial or spectral resolution for detailed analysis of the reactions taking place in catalytic microreactors. Synchrotron-based infrared microspectroscopy at the ALS, with a diffraction-limited infrared beam diameter of less than 10 μm, can overcome this hurdle. And while previous studies used synchrotron microspectroscopy to characterize simple gas-phase catalytic reactions, in this work the researchers obtained detailed mechanistic information about multistep, multiphase catalytic processes within a flow reactor under reaction conditions.
With this new method, researchers can effectively watch an entire catalytic movie, from reactants to product formation, instead of only snapshots of the catalytic process. Previously, in most cases, chemists had to extrapolate information on the reaction process based on analysis of the final product. With this novel infrared mapping technique, they don’t have to guess what happened in the first scene based on what they saw in the final scene; instead they can now directly watch a high-resolution movie of the entire process.
At ALS Beamline 1.4, the researchers collected infrared absorption spectra along the length of the flow reactor with 15-μm spatial resolution and 4-cm–1 spectral resolution. Analysis of this data yielded information about the kinetic evolution of the reaction—answering questions about the various stages of the multistep process, which steps were rate-determining in each stage, and what reactant flow rate maximized production of the desired product.
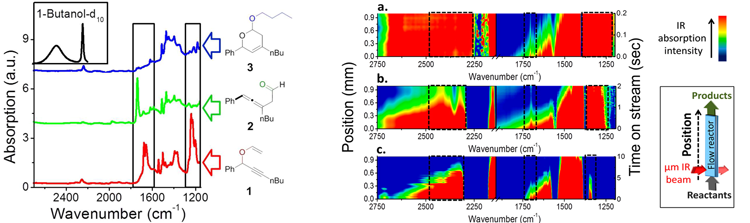
In following the reactions step by step, the researchers also discovered that the catalytic reaction they were observing was completed within the first 5% of the flow reactor’s volume, which meant that the remaining 95% of the reactor, though packed with catalyst, did not contribute to the catalytic process. X-ray absorption spectroscopy at the gold L3 edge was performed at Beamline 10.3.2, revealing that the catalytically active Au(III) species gradually decreased due to catalyst reduction to Au(0). Based on this result, the researchers were able to minimize the volume of the flow reactor and the amount of catalyst by an order of magnitude without deteriorating the catalytic reactivity.
While the infrared microspectroscopy technique employed in this study allowed one-dimensional mapping of a catalytic reaction along the path of the flow reactor, the actual flow reactor is three dimensional. The team is now exploring techniques that would permit two- and three-dimensional mapping of catalytic reactions. Multidimensional imaging will give the ability to know where exactly inside the volume of the flow reactor the catalytic reaction takes place. This will provide advanced tools for better understanding and optimization of the catalytic reaction.
Contacts: Gabor Somorjai and Dean Toste
Research conducted by: E. Gross, X.-Z. Shu, S. Alayoglu, F.D. Toste, and G.A. Somorjai (Univ. of California, Berkeley), and H.A. Bechtel and M.C. Martin (ALS).
Research funding: U.S. Department of Energy (DOE), Office of Basic Energy Sciences (BES). Operation of the ALS is supported by DOE BES.
Publication about this research: E. Gross, X.-Z. Shu, S. Alayoglu, H.A. Bechtel, M.C. Martin, F.D. Toste, and G.A. Somorjai, “In Situ IR and X-ray High Spatial-Resolution Microspectroscopy Measurements of Multistep Organic Transformation in Flow Microreactor Catalyzed by Au Nanoclusters,” J. Am. Chem. Soc. 136, 3624 (2014). doi: 10.1021/ja412740p
ALS SCIENCE HIGHLIGHT #296