Lysine is an important amino acid that must be supplied in our diets, as our bodies can’t produce it. Low lysine content in crops such as cereals and legumes results in protein-energy malnutrition in 30% of the population of the developing world. The engineering of crops with higher lysine levels is a goal that requires not only lysine overproduction in plants but also the concurrent disruption of lysine breakdown. However, the biochemical processes that catabolize lysine in plants haven’t been fully understood. In particular, there’s been no consensus about the steps in the process following the formation of a chemical intermediate known as 2-oxoadipate (2OA).
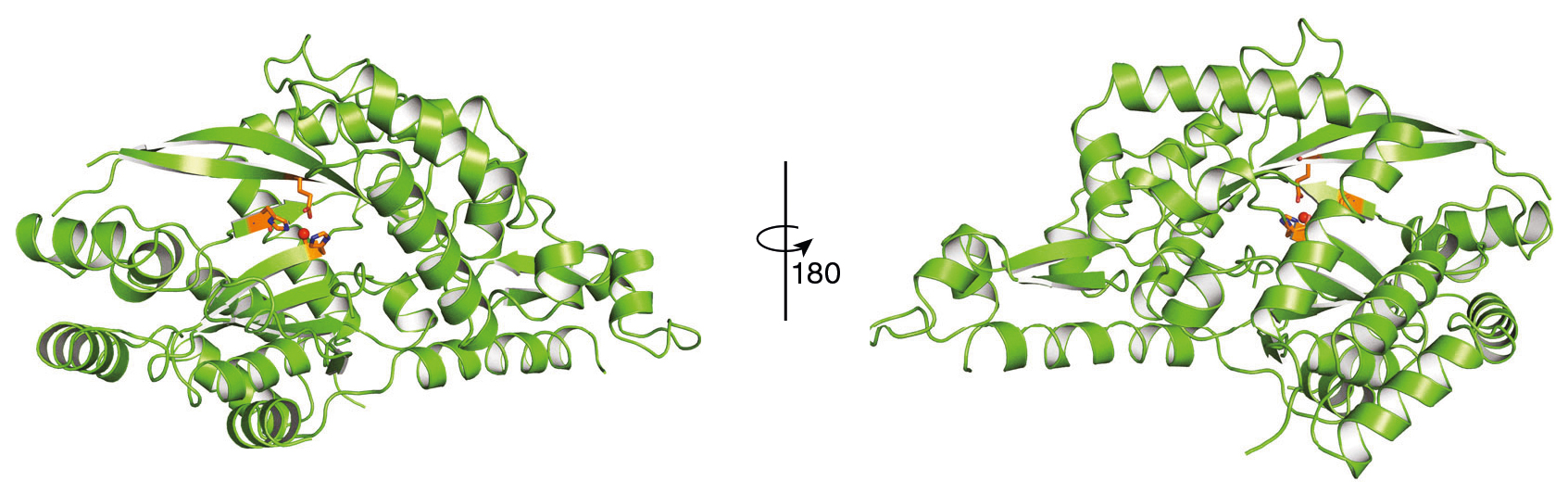
Recently, researchers from the Joint BioEnergy Institute (JBEI) described a novel catabolic pathway in which 2OA is converted into its product, D-2-hydroxyglutarate (2HG), via the linking enzyme, hydroxyglutarate synthase (HglS). To better understand this reaction, the researchers performed protein crystallography on HglS at ALS Beamlines 5.0.2 and 8.2.2, part of the Berkeley Center for Structural Biology (BCSB). Based on the results, the researchers proposed a detailed catalytic mechanism for HglS involving a metallic cofactor, most likely Fe(II). Notably, HglS is a DUF 1338 protein (“DUF” proteins are characterized by “domains of unknown function”), and the elucidation of its function is a first for a member of the DUF 1338 family.
With this final piece of the lysine metabolic process in place, the researchers hope that a more complete understanding of lysine catabolism will aid in the successful development of stable high-lysine crops to combat malnutrition globally.
M.G. Thompson, J.M. Blake-Hedges, J.H. Pereira, J.A. Hangasky, M.S. Belcher, W.M. Moore, J.F. Barajas, P. Cruz-Morales, L.J. Washington, R.W. Haushalter, C.B. Eiben, Y. Liu, W. Skyrud, V.T. Benites, T.P. Barnum, E.E.K. Baidoo, H.V. Scheller, M.A. Marletta, P.M. Shih, P.D. Adams, and J.D. Keasling, “An iron (II) dependent oxygenase performs the last missing step of plant lysine catabolism,” Nat. Commun. 11, 2931 (2020), doi: 10.1038/s41467-020-16815-3.
Adapted from the JBEI news article, “Finding the missing step of an important molecular process.”