Lignin is a polymer that permeates plant cell walls. Although beneficial to the plant, the lignin must be chemically broken down in a costly pretreatment step before the sugars inside can be released and fermented into useful chemicals and fuels. Previous attempts to silence lignin-producing genes resulted in weak plants with a lower sugar yield.
In this work, researchers focused instead on an enzyme called HCT that plays a key role in lignin synthesis and was known to be potentially “promiscuous,” binding with a variety of molecules. By swapping the usual lignin-producing molecule at the binding site with lignin-inhibiting molecules in initial tests with a model plant, they were able to decrease lignin content by 30% while upping sugar production.
At the ALS, protein crystallography studies at Beamlines 8.2.1 and 8.2.2 of the HCT from similarly modified switchgrass confirmed that the new molecules attached to the enzyme at the site normally occupied by the lignin-producing molecule, with similar affinities to the binding site.
This technique promises to be much more “tunable” than the gene-silencing method of reducing plant lignin, which decreases lignin everywhere in a plant and throughout its lifespan. With this new approach, the goal is to tune the process so that lignin is reduced in a plant where we want it reduced, such as in tissues that produce thick cell walls, and when we want it reduced, such as later in a plant’s development.
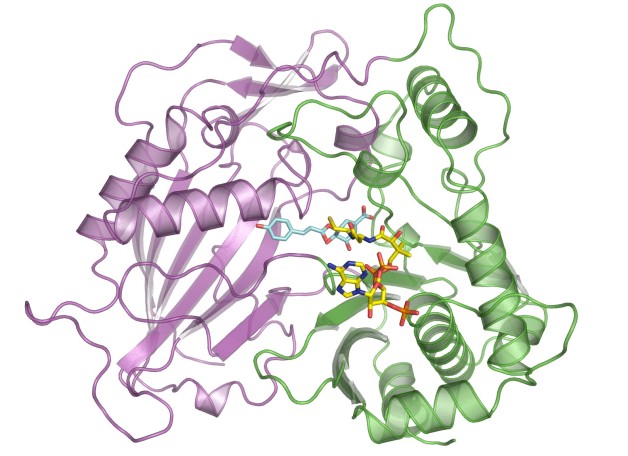
Work performed at ALS Beamlines 8.2.1 and 8.2.2 (Berkeley Center for Structural Biology).
Aymerick Eudes, Jose H. Pereira, Sasha Yogiswara, George Wang, Veronica Teixeira Benites, Edward E.K. Baidoo, Taek Soon Lee, Paul D. Adams, Jay D. Keasling, and Dominique Loqué, “Exploiting The Substrate Promiscuity of Hydroxycinnamoyl-CoA:shikimate Hydroxycinnamoyl Transferase to Reduce Lignin,” Plant Cell Physiol. 57, 568 (2016).