SCIENTIFIC ACHIEVEMENT
X-ray crystallography of a membrane protein provided a structural understanding of how a single mutation can result in periodic muscle paralysis.
SIGNIFICANCE AND IMPACT
The results suggest possible drug designs that could provide relief to patients with a genetic disorder that causes them to be overcome suddenly with profound muscle weakness.
Hypokalemic periodic paralysis
A rare genetic disorder called hypokalemic periodic paralysis (hypoPP) causes sudden, profound muscle weakness in people who occasionally exhibit low levels of potassium in their blood, or hypokalemia. When a patient is hypokalemic, hypoPP affects the function of the muscles responsible for skeletal movement. The disease has been known to stem from mutations in certain membrane proteins that channel and regulate the flow of sodium into cells. Exactly how the mutation affects the proteins’ function, however, was not known.
In earlier work, researchers from the Catterall Lab at the University of Washington had solved the structure of a sodium channel called NavAb from a prokaryote (single-celled organism). As a next step, the group decided to see if NavAb could serve as a model for studying the mutations that cause hypoPP in humans (eukaryotes), with the goal of finding a way to prevent or treat this disorder.
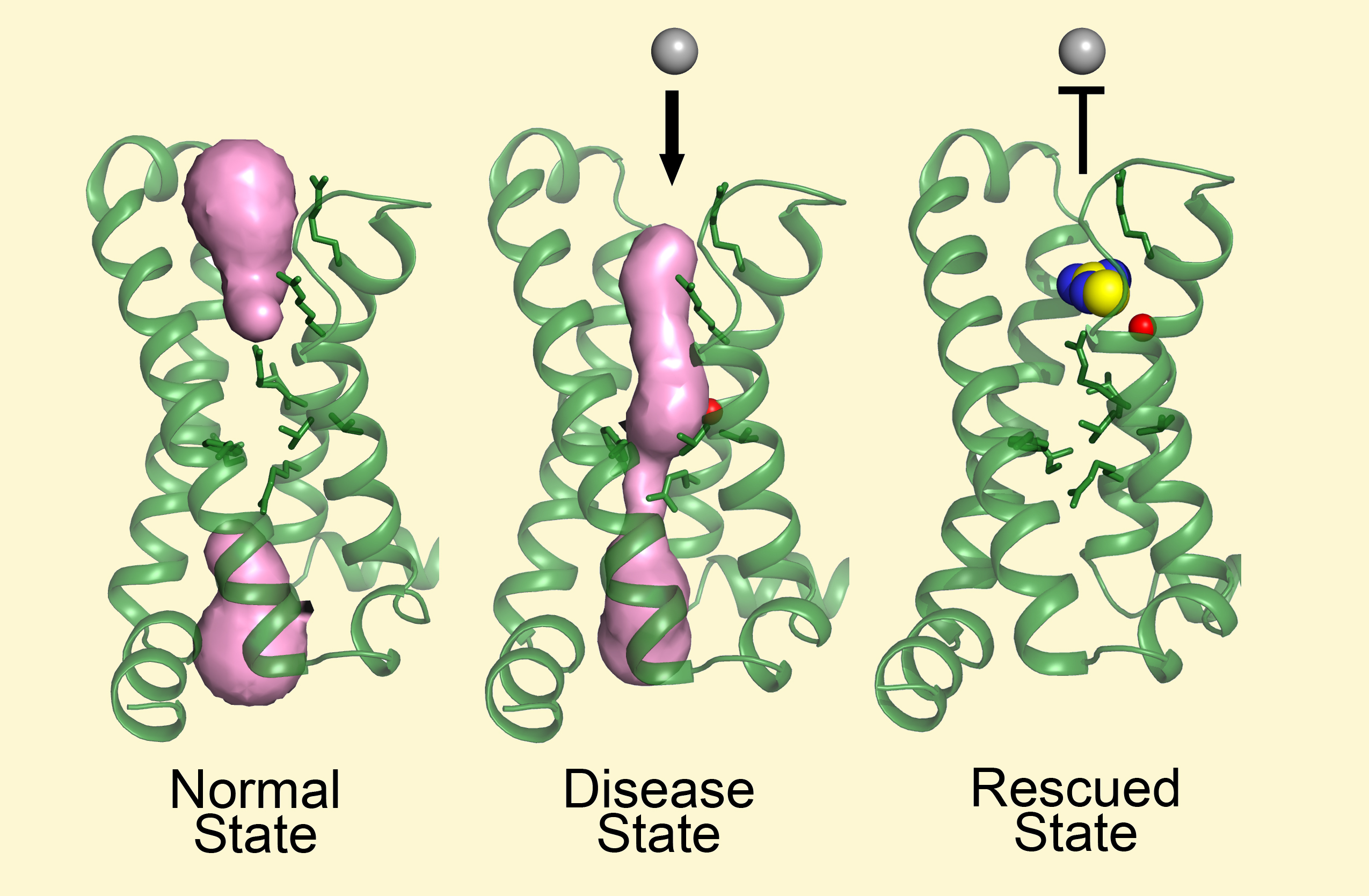
A leak in the pipe?
In a resting state, muscle-cell membranes keep potassium ions and sodium ions separated, inside and outside the cell, respectively, creating a voltage across the membrane. A chemical signal from a nerve cell sets off a cascade of events that results in sodium ions flowing into the cell, changing the membrane potential and and ultimately triggering muscle contraction.
In theory, the hypoPP mutation creates a small hole through the center of the channel protein’s voltage-sensing domain (VSD). Sodium ions can continuously leak through this opening, causing sustained membrane depolarization, which paralyzes the muscle. Under ordinary circumstances, ion pumps that exchange potassium for sodium compensate for this by pumping sodium out while pumping potassium in. But when extracellular potassium concentrations fall below normal, this is no longer possible.
A solution crystallizes
In this study, the researchers compared crystallographic data from mutated and nonmutated (wild-type) NavAb sodium channels. The results were combined with electrophysiological data on the channels’ gating pore currents. In addition, the researchers crystallized one NavAb mutant from solution with a compound called guanidinium, which has been known to provide relief from symptoms of hypoPP.
The structures were solved using protein crystallography data collected at ALS Beamlines 8.2.1 and 8.2.2, part of the Berkeley Center for Structural Biology. The very stable and bright ALS beam enabled the collection of high-quality, high-resolution data, and the ability to screen hundreds of crystals efficiently through remote access was crucial to the success of this work.
Closing the gate
One of the mutant structures clearly revealed a continuous hole in the VSD, capable of causing an abnormal sodium leak. Because the measured gating pore current in the mutant was very small, the researchers were surprised that they could see such an obvious hole in the VSD crystal structure. The structures also showed that guanidinium can indeed block the pore created by the mutation and stop the sodium leak without altering the voltage sensor’s ability to function.
This work not only provides a structural basis for understanding how the gating pore current causes periodic paralysis, it may also provide molecular templates for design and development of drugs that would mimic guanidinium, block gating pore current, and provide symptomatic relief of periodic paralysis.
Contact: Daohua Jiang
Researchers: D. Jiang, T.M. Gamal El-Din, P. Lu, and W.A. Catterall (University of Washington); C. Ing and R. Pomès (Hospital for Sick Children Toronto and University of Toronto); and N. Zheng (University of Washington and Howard Hughes Medical Institute).
Funding: National Institutes of Health, Howard Hughes Medical Institute, and Canadian Institutes of Health. Operation of the ALS is supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences Program (DOE BES).
Publication: D. Jiang, T.M. Gamal El-Din, C. Ing, P. Lu, R. Pomès, N. Zheng, and W.A. Catterall, “Structural basis for gating pore current in periodic paralysis,” Nature 557, 590 (2018), doi:10.1038/s41586-018-0120-4.
Adapted from the University of Washington press release, “Study reveals why a rare disorder causes sudden paralysis.”
ALS SCIENCE HIGHLIGHT #381