Detecting viral infection can be tricky for a cell. Viruses rely so much on host cells that, during infection, there is little machinery that’s unique to the virus—and few things to trigger antiviral alarm systems. One way that cells deal with this is to recognize viral RNA or DNA.
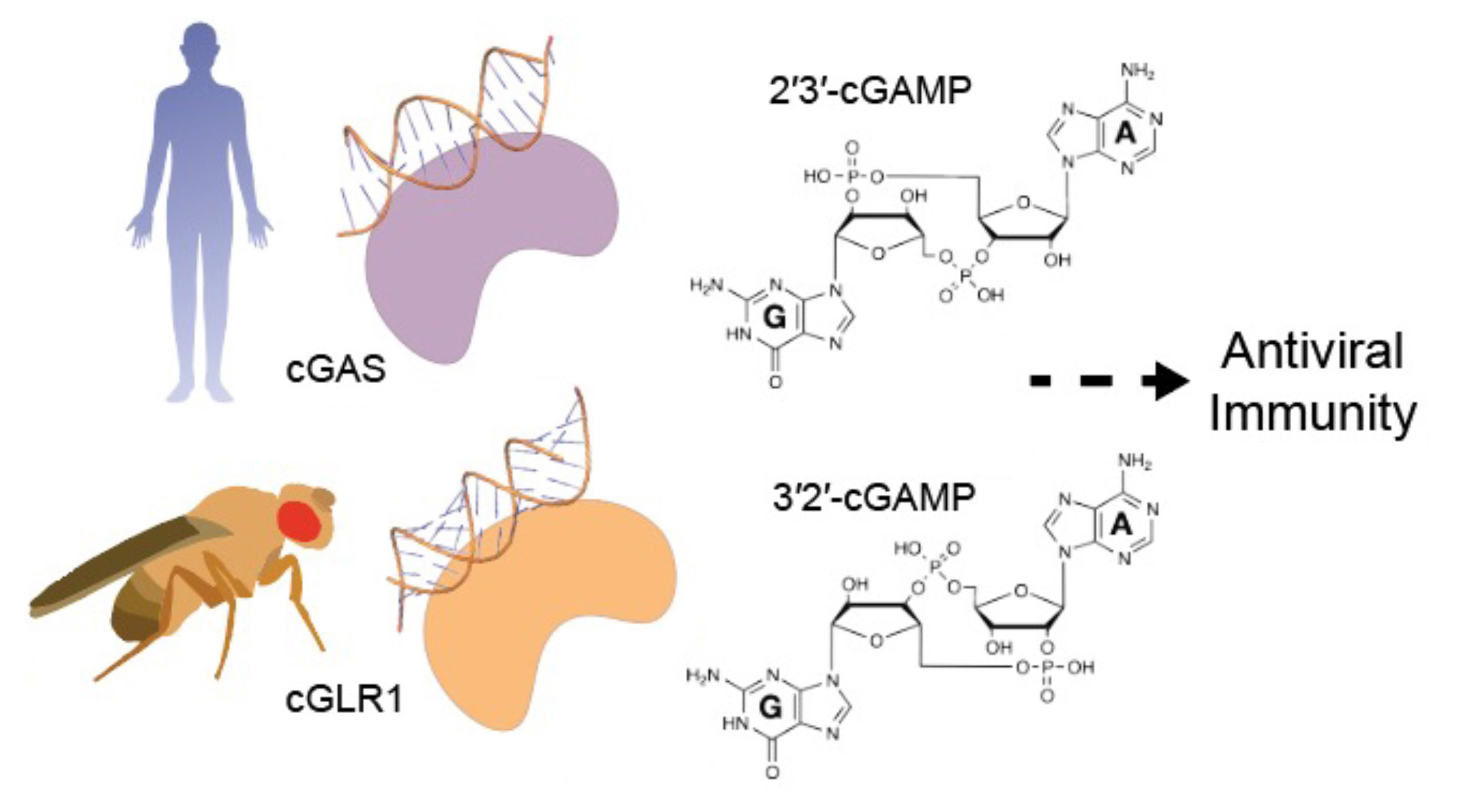
In humans, one sentinel for viral DNA is an enzyme called cGAS. It synthesizes a signaling molecule, cGAMP, that alerts cells to viral infection. Now, a new study has found that cGAS is part of a larger family of evolutionarily related proteins (homologs) called “cGAS-like receptors” (cGLRs).
“We’ve known that most animals—including humans—encode predicted homologs of cGAS,” said Philip Kranzusch, a researcher at Harvard Medical School and the Dana-Farber Cancer Institute. “But we didn’t understand how these enzymes function or if they had a role in immunity.”
In this work, researchers tackled the question by comparing the structures of human and insect cGLRs using protein crystallography at Advanced Light Source Beamlines 5.0.1 and 8.2.2. The results showed that cGLRs differ from cGAS at their DNA-binding surfaces. The researchers then used a biochemical screen to identify a new antiviral signaling pathway controlled by a cGLR dubbed cGLR1, in the fly species Drosophila melanogaster.
Instead of recognizing DNA, cGLR1 responds to RNA. However, the real surprise came when the team discovered that cGLR1 synthesizes a unique form of cGAMP with a distinct chemical backbone.
“This is the first time we’ve found cGLRs signaling through a different messenger—and we think it specifically evolved in flies, perhaps as a strategy to shield this signaling system from viral antagonism,” said the paper’s lead author, Kailey Slavik, a graduate student in Kranzusch’s lab. “It turns out that in flies, just like in humans, the receptor of this messenger is specifically adapted to see the ‘in-house’ flavor of cGAMP.”
While this study reveals that structural variation enables cGLRs to respond to diverse pathogen signatures through different messengers, most animal cGLR-signaling pathways remain uncharacterized. “There’s so much left to be discovered about how cGLRs control immune signaling in humans and other animals,” said Kranzusch. “Kailey’s research in the lab is just the beginning.”
K.M. Slavik, B.R. Morehouse, A.E. Ragucci, W. Zhou, X. Ai, Y. Chen, L. Li, Z. Wei, H. Bähre, M. König, R. Seifert, A.S.Y. Lee, H. Cai, J.-L. Imler, and P.J. Kranzusch, “cGAS-like receptors sense RNA and control 3′2′-cGAMP signalling in Drosophila,” Nature 597, 109 (2021), doi:10.1038/s41586-021-03743-5.